Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (1): 18-40.DOI: 10.6023/cjoc202306003 Previous Articles Next Articles
REVIEWS
贾小英a,b, 普佳霞a,b, 韩丽荣a,b, 李清寒a,b,*( )
)
收稿日期:2023-06-02
修回日期:2023-07-28
发布日期:2023-08-30
基金资助:
Xiaoying Jiaa,b, Jiaxia Pua,b, Lirong Hana,b, Qinghan Lia,b( )
)
Received:2023-06-02
Revised:2023-07-28
Published:2023-08-30
Contact:
*E-mail: Supported by:Share
Xiaoying Jia, Jiaxia Pu, Lirong Han, Qinghan Li. Research Progress in the Synthesis of Benzo[d]pentamembered Heterocyclic Thioethers Containing Two Heteroatoms[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 18-40.
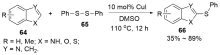
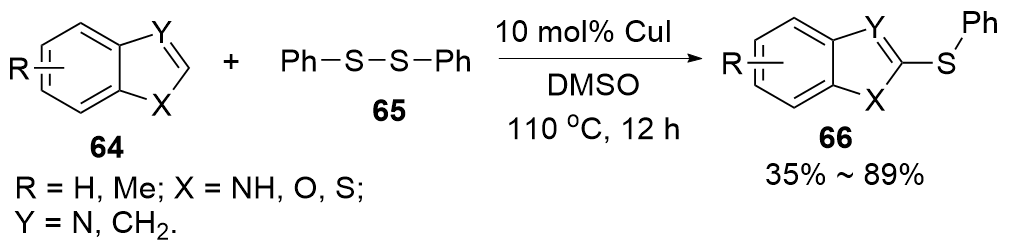
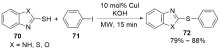
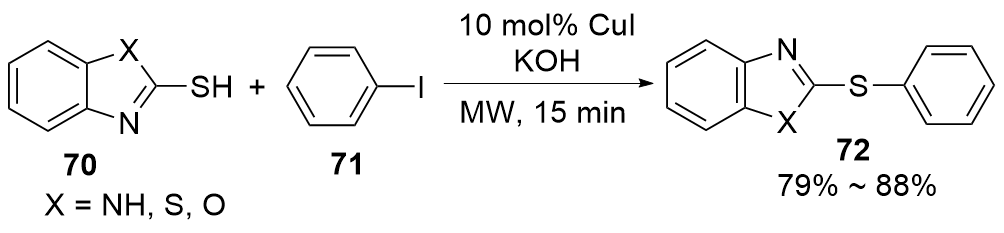
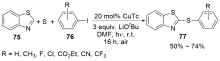
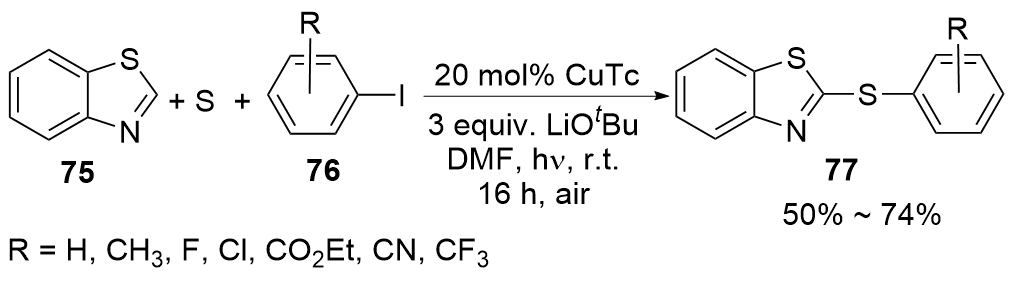
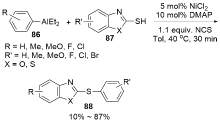
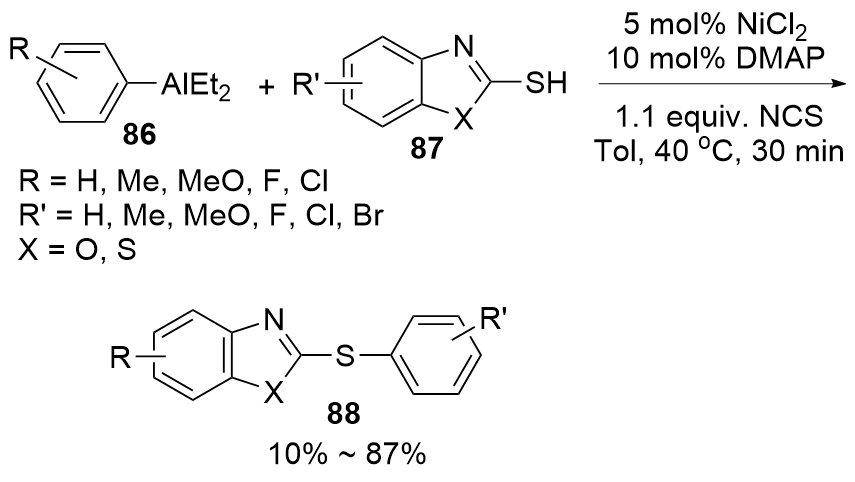
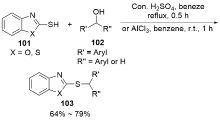
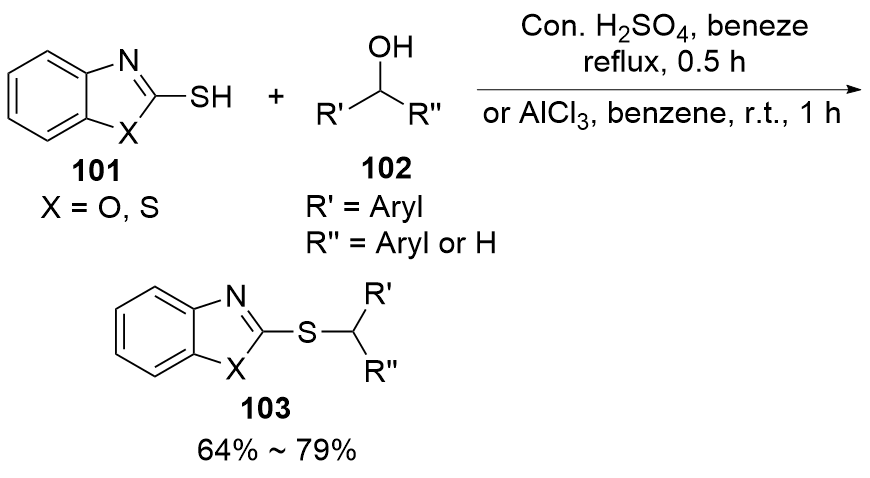
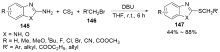
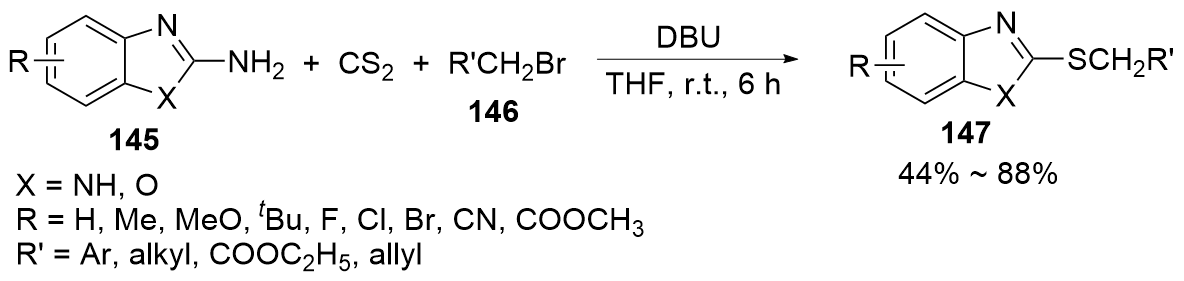
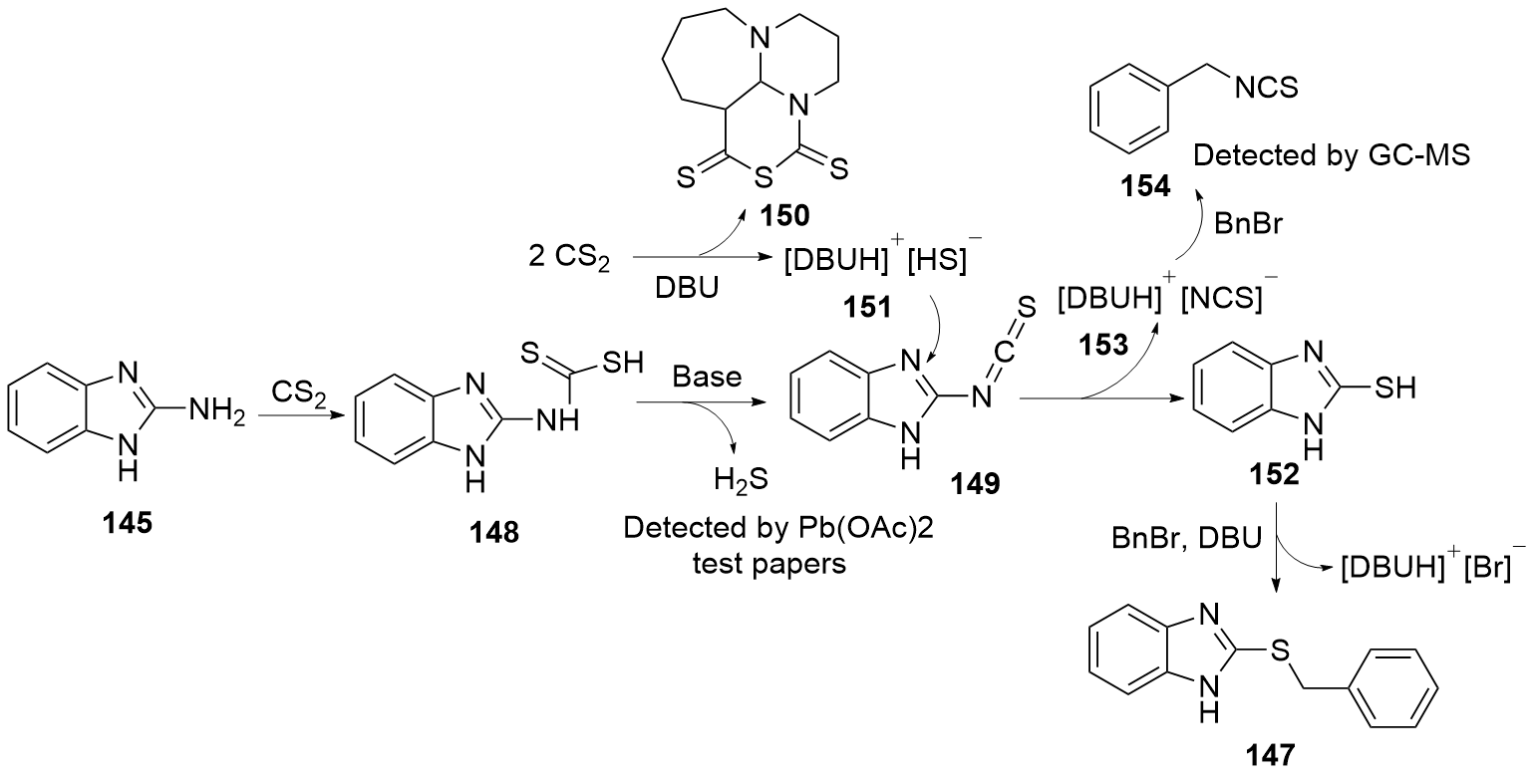

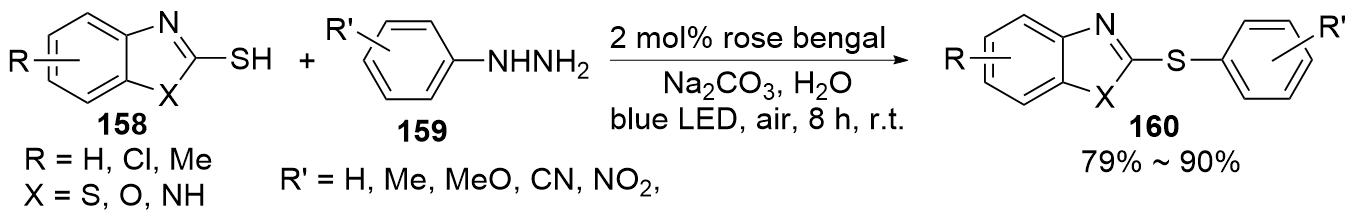
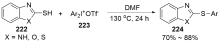
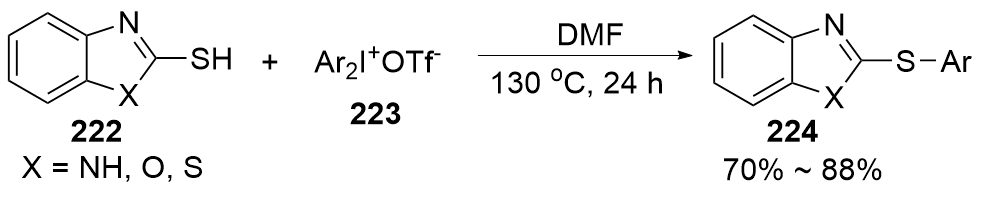
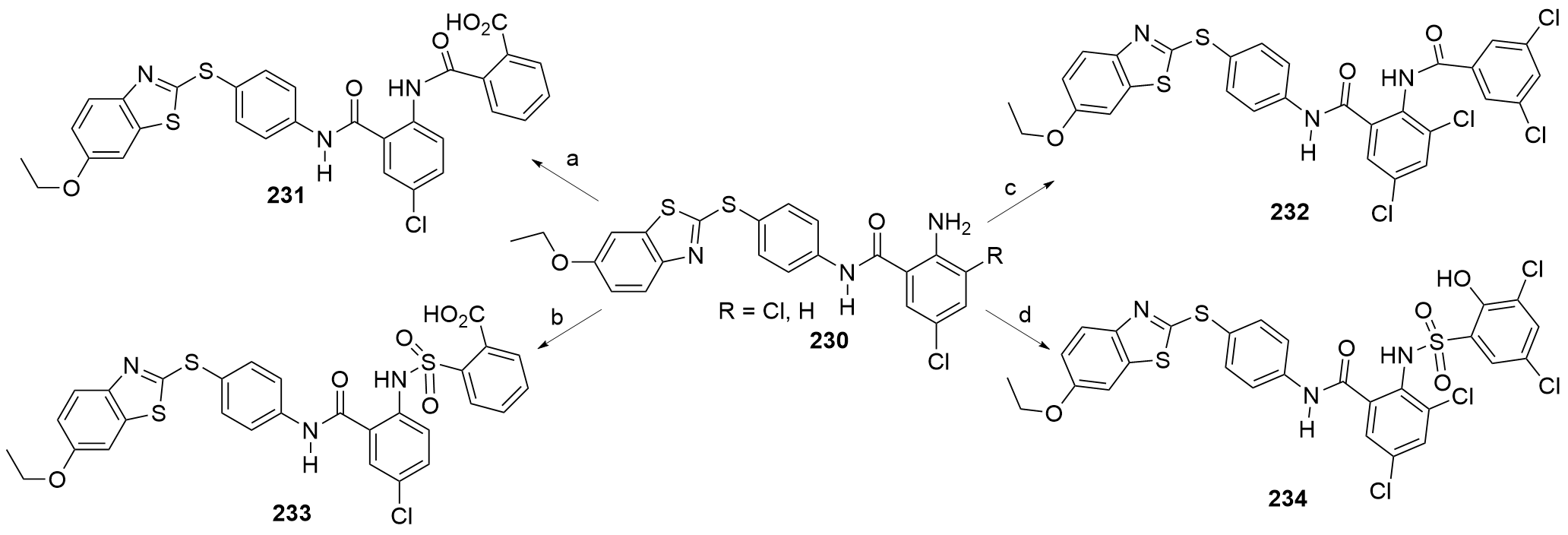
| [1] |
Qin, Z.; Kastrati, I.; Chandrasena, R. E. P.; Liu, H.; Yao, P.; Petukhov, P. A.; Bolton, J. L.; Thatcher, G. R. J. J. Med. Chem. 2007, 50, 2682.
doi: 10.1021/jm070079j |
| [2] |
Bagley, M. C.; Davis, T.; Dix, M. C.; Rokicki, M.; Kipling, D. Bioorg. Med. Chem. Lett. 2007, 17, 5107.
doi: 10.1016/j.bmcl.2007.07.016 |
| [3] |
Beletskaya, I. P.; Ananikov, V. P. Chem. Rev. 2011, 111, 1596.
doi: 10.1021/cr100347k |
| [4] |
Ubale, A. U.; Bhute, M. V.; Malpe, G. P.; Raut, P. P.; Chipade, K. S.; Ibrahim, S. G. J. Sadui Chem. Soc. 2016, 20, 227.
|
| [5] |
Akhtar, T.; Hameed, S.; Al-Masoudi, N. A.; Loddo, R.; Colla, P. Acta Pharm. 2008, 58, 135.
|
| [6] |
Singh, M.; Singh, S. K.; Gangwar, M.; Nath, G.; Singh, S. K. RSC Adv. 2014, 4, 19013.
doi: 10.1039/C4RA02649G |
| [7] |
Martino, G. D.; Regina, G. L.; Coluccia, A.; Edler, M. C.; Barbera, M. C.; Brancale, A.; Silvestri, R. J. Med. Chem. 2004, 47, 6120.
doi: 10.1021/jm049360d |
| [8] |
Huang, S. T.; Hsei, I. J.; Chen, C. Bioorg. Med. Chem. 2006, 14, 6106.
doi: 10.1016/j.bmc.2006.05.007 |
| [9] |
Kucuksayan, E.; Ozben, T. Curr. Top. Med. Chem. 2017, 17, 907.
doi: 10.2174/1568026616666160927155515 |
| [10] |
Nielsen, S. F.; Nielsen, E. O.; Olsen, G. M.; Liljefors, T.; Peters, D. J. Med. Chem. 2000, 43, 2217.
doi: 10.1021/jm990973d |
| [11] |
Alcaraz, M. L.; Atkinson, S.; Cornwall, P.; Foster, A. C.; Gill, D. M.; Humphries, L. A.; Keegan, P. S.; Kemp, R.; Merifield, E.; Nixon, R. A.; Noble, A. J.; O’Beirne, D.; Patel, Z. M.; Perkins, J.; Rowan, P.; Sadler, P.; Singleton, J. T.; Tornos, J.; Watts, A. J.; Woodland, I. A. Org. Process Res. Dev. 2005, 9, 555.
doi: 10.1021/op0500483 |
| [12] |
Kumat, S.; Engman, L. J. Org. Chem. 2006, 71, 5400.
doi: 10.1021/jo060690a |
| [13] |
Azam, M. A.; Suresh, B. Sci. Pharm. 2012, 80, 789.
doi: 10.3797/(ISSN)0036-8709 |
| [14] |
(a) Tang, X. C.; Zhao, Y. W. Mini-Rev. Org. Chem. 2021, 18, 902.
doi: 10.2174/1570193X17999201113142442 |
|
(b) Zhang, B. X.; Kang, Y. Q.; Shi, R. X. Chin. J. Org. Chem. 2016, 36, 1814 (in Chinese).
doi: 10.6023/cjoc201602021 |
|
|
(张变香, 亢永强, 史瑞雪, 有机化学, 2016, 36, 1814.)
|
|
| [15] |
(a) Chen, Z. W.; Bai, R.; Annamalai, P.; Badsara, S. S.; Lee, C. F. New J. Chem. 2022, 46, 15.
doi: 10.1039/D1NJ04662D |
|
(b) Sundaravelu, N. S.; Sangeetha, S.; Sekar, G. Org. Biomol. Chem. 2021, 19, 1459.
doi: 10.1039/D0OB02320E |
|
| [16] |
(a) Cacchi, S.; Fabrizi, G. Chem. Rev. 2005, 105, 2873.
doi: 10.1021/cr040639b |
|
(b) Behera, P. K.; Choudhury, P.; Behera, P.; Swain, A.; Pradhan, A. K.; Rout, L. ChemistrySelect 2022, 7, e202202919.
|
|
| [17] |
(a) Cong, M.; Fan, Y.; Raimundo, J. M.; Xia, Y.; Liu, Y.; Quelever, G.; Qu, F. Q.; Peng, L. Chem.-Eur. J. 2013, 19, 17267.
doi: 10.1002/chem.v19.51 |
|
(b) Cong, M.; Fan, Y.; Raimundo, J. M.; Tang, J.; Peng, L. Org. Lett. 2014, 16, 4074.
doi: 10.1021/ol501600k |
|
| [18] |
Zhang, Z. X.; Chen, P. H.; Liu, G. S. Chem. Soc. Rev. 2022, 51, 1640.
doi: 10.1039/D1CS00727K |
| [19] |
Murru, S.; Ghosh, H.; Sahoo, S. K.; Patel, B. K. Org. Lett. 2009, 11, 4254.
doi: 10.1021/ol9017535 |
| [20] |
Murru, S.; Mondal, P.; Yella, R.; Patel, B. K. Eur. J. Org. Chem. 2009, 2009, 5406.
doi: 10.1002/ejoc.v2009:31 |
| [21] |
Fukuzawa, S. I.; Shimizu, E.; Atsuumi, Y.; Haga, M.; Ogata, K. Tetrahedron Lett. 2009, 50, 2374.
doi: 10.1016/j.tetlet.2009.02.214 |
| [22] |
Ranjit, S.; Lee, R.; Heryadi, D.; Shen, C.; Wu, J.; Zhang, P.; Hang, K. W.; Liu, X. J. Org. Chem. 2011, 76, 8999.
doi: 10.1021/jo2017444 |
| [23] |
Xu, H. J.; Zhao, Y. Q.; Feng, T.; Feng, Y. S. J. Org. Chem. 2012, 43, 2878.
|
| [24] |
Wang, X. W.; Li, Y. J.; Yuan, Y. Synthesis 2013, 45, 1247.
doi: 10.1055/s-00000084 |
| [25] |
Liu, Y. Y.; Huang, B.; Cao, X.; Wu, D.; Wan, J. P. RSC Adv. 2014, 4, 37733.
doi: 10.1039/C4RA07187E |
| [26] |
Liu, X.; Dong, Z. B. J. Org. Chem. 2019, 84, 11524.
doi: 10.1021/acs.joc.9b01370 |
| [27] |
Savarin, C.; Srogl, J.; Liebeskind, L. S. Org. Lett. 2002, 4, 4309.
doi: 10.1021/ol026948a |
| [28] |
Shi, L.; Liu, X.; Zhang, H.; Jiang, Y.; Ma, D. W. J. Org. Chem. 2011, 76, 4200.
doi: 10.1021/jo200535e |
| [29] |
Dai, C.; Xu, Z. Q.; Huang, F.; Yu, Z. K.; Gao, Y. F. J. Org. Chem. 2012, 77, 4414.
doi: 10.1021/jo202624s |
| [30] |
He, G. Z.; Huang, Y.; Tong, Y.; Zhang, J.; Zhao, D.; Zhou, S. L.; Han, S. Q. Tetrahedron Lett. 2013, 54, 5318.
doi: 10.1016/j.tetlet.2013.07.096 |
| [31] |
Li, X.; Yuan, T.; Yang, Y.; Chen, J. M. Tetrahedron 2014, 70, 9652.
doi: 10.1016/j.tet.2014.10.075 |
| [32] |
Zhang, B. X.; Chen, K.; Yang, L. H.; Xu, Y. T.; Zhang, R. J.; Zhang, L. N.; Shi, R. X. Chin. J. Org. Chem. 2015, 35, 905 (in Chinese).
doi: 10.6023/cjoc201410009 |
|
(张变香, 陈凯, 杨丽花, 许钰涛, 张瑞杰, 张利娜, 史瑞雪, 有机化学, 2015, 35, 905. )
|
|
| [33] |
Zhang, B. X.; Yang, L. H.; Shi, R. X.; Kang, Y. Q. Chin. J. Org. Chem. 2016, 36, 352 (in Chinese).
doi: 10.6023/cjoc201509026 |
|
(张变香, 杨丽花, 史瑞雪, 亢永强, 有机化学, 2016, 36, 352.)
|
|
| [34] |
Gandeepan, P.; Mo, J.; Ackermann, L. Chem. Commun. 2017, 53, 5906.
doi: 10.1039/C7CC03107F |
| [35] |
Rosario, A. R.; Casola, K. K.; Oliveira, C. E. S.; Zeni, G. Adv. Synth. Catal. 2013, 355, 2960.
doi: 10.1002/adsc.v355.14/15 |
| [36] |
Satish, G.; Reddy, K. H. V.; Ramesh, K.; Shankar, J.; Nageswar, Y. V. D. Eur. J. Chem. 2014, 5, 291.
doi: 10.5155/eurjchem.5.2.291-295.861 |
| [37] |
Luo, R. Q.; Guo, S. P.; Xiao, H. L.; Li, Q. H. Tetrahedron 2022, 103, 132564.
doi: 10.1016/j.tet.2021.132564 |
| [38] |
Rafique, J.; Saba, S.; Frizon, T. E. A.; Braga, A. L. ChemistrySelect 2018, 3, 328.
doi: 10.1002/slct.v3.1 |
| [39] |
Cheng, J. H.; Ramesh, C.; Kao, H. L.; Wang, Y. J.; Chan, C. C.; Lee, C. F. J. Org. Chem. 2012, 77, 10369.
doi: 10.1021/jo302088t |
| [40] |
Yonova, I. M.; Osborne, C. A.; Morrissette, N. S.; Jarvo, E. R. J. Org. Chem. 2014, 79, 1947.
doi: 10.1021/jo402586v |
| [41] |
Goyal, M.; Singh, P.; Alam, A.; Das, S. K.; Iqbal, M. S.; Dey, S.; Bindu, S.; Pal, C.; Das, S. K.; Panda, G.; Bandyopadhyay, U. Biol. Med. 2012, 53, 129.
doi: 10.3181/00379727-53-14217P |
| [42] |
Varun, B. V.; Prabhu, K. R. J. Org. Chem. 2014, 79, 9655.
doi: 10.1021/jo501793q |
| [43] |
Yuan, C.; Li, J.; Li, P. F. ACS Omega 2018, 3, 6820.
doi: 10.1021/acsomega.8b01207 |
| [44] |
Miao, C. X.; Zhuang, H. F.; Wen, Y. T.; Han, F.; Yang, Q. F.; Yang, L.; Li, Z.; Xia, C. G. Eur. J. Org. Chem. 2019, 2019, 3012.
doi: 10.1002/ejoc.v2019.19 |
| [45] |
Truong, T. S.; Retailleau, P.; Nguyen, T. B. Asian J. Org. Chem. 2022, 11, e202100751.
|
| [46] |
Klimešova, V.; Koči, J.; Pour, M.; Stachel, J.; Waisser, K.; Kaustova, J. Eur. J. Med. Chem. 2002, 37, 409.
doi: 10.1016/S0223-5234(02)01342-9 |
| [47] |
Zhang, C. R.; Wang, L.; Ge, Y. L.; Ju, X. L. Chin. J. Org. Chem. 2007, 27, 1432 (in Chinese).
|
|
(张成仁, 王柳, 葛燕丽, 巨修练, 有机化学, 2007, 27, 1432.)
|
|
| [48] |
De, S. K.; Chen, L. H.; Stebbins, J. L.; Machleidt, T.; Riel-Mehan, M.; Dahl, R.; Chen, V.; Yuan, H. B.; Barile, E.; Emdadi, A.; Murphy, R.; Pellecchia, M. Bioorg. Med. Chem. 2009, 17, 2712.
|
| [49] |
Cruz-Gonzalez, D. Y.; Gonzalez-Olvera, R.; Angeles-Beltran, D.; Negron-Silva, G. E.; Santillan, R. Synthesis 2013, 45, 3281.
doi: 10.1055/s-00000084 |
| [50] |
Zheng, L. Y.; Mei, W. J.; Zou, X. Y.; Zhong, Y. M.; Wu, Y. Y.; Deng, L.; Wang, Y. H.; Yang, B. N.; Guo, W. J. Org. Chem. 2023, 88, 2190.
doi: 10.1021/acs.joc.2c02610 |
| [51] |
Zhang, Z.; Wu, H. H.; Tan, Y. J. RSC Adv. 2013, 3, 16940.
doi: 10.1039/C3RA43252A |
|
(b) Fu, Z.; Yuan, W.; Chen, N.; Yang, Z.; Xu, J. Green Chem. 2018, 20, 4484.
doi: 10.1039/C8GC02261E |
|
| [52] |
Cano, H. N.; Ballari, M. S.; López, A. G.; Santiago, A. N. J. Agric. Food Chem. 2015, 63, 3681.
doi: 10.1021/acs.jafc.5b00150 |
| [53] |
Kibriya, G.; Mondal, S.; Hajra, A. Org. Lett., 2018, 20, 7740.
doi: 10.1021/acs.orglett.8b03549 |
| [54] |
Zhang, S. B.; Liu, X.; Gao, M. Y.; Dong, Z. B. J. Org. Chem. 2018, 83, 14933.
doi: 10.1021/acs.joc.8b02136 |
| [55] |
(a) Zeng, M. T.; Xu, W.; Liu, X.; Chang, C. Z.; Zhu, H.; Dong, Z. B. Eur. J. Org. Chem. 2017, 2017, 6060.
doi: 10.1002/ejoc.v2017.40 |
|
(b) Dong, Z. B.; Liu, X.; Bolm, C. Org. Lett. 2017, 19, 5916.
doi: 10.1021/acs.orglett.7b02911 |
|
| [56] |
Venkata Subbaiah, B.; Sree Ganesh, K. K.; Prakash, L.; Subramanyam Reddy, K. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1243.
doi: 10.1080/10826076.2012.685921 |
| [57] |
Zhou, G.; Guan, Y. Heterocycl. Commun. 2016, 22, 17.
doi: 10.1515/hc-2015-0101 |
| [58] |
Ballari, M. S.; Cano, N. H.; Lopez, A. G.; Wunderlin, D. A.; Feresin, G. E.; Santiago, A. N. J. Agric. Food Chem. 2017, 65, 10325.
doi: 10.1021/acs.jafc.7b04130 |
| [59] |
Ramanjaneyulu, B. T.; Vidyacharan, S.; Ahn, G.; Kim, D. React. Chem. Eng. 2020, 5, 849.
doi: 10.1039/D0RE00038H |
| [60] |
Rambo, R. S.; Waldow, E. C.; Abaddi, B. L.; Silveira, M. D.; Dadda, A. S.; Sperotto, N.; Bizarro, C. V.; Basso, L. A.; Machado, P. J. Braz. Chem. Soc. 2021, 32, 1413.
|
| [61] |
Kalogirou, A. S.; Koutentis, P. A. Molbank 2022, 2022, M1321. https://doi.org/10.3390/M1321.
doi: 10.3390/M1321 |
| [62] |
Wang, D.; Liu, Z.; Wang, Z.; Ma, X.; Yu, P. Green Chem. 2019, 21, 157.
doi: 10.1039/C8GC03072C |
| [63] |
Wang, F. Y.; Chen, Z. C.; Zheng, Q. G. J. Chem. Res. 2004, 2004, 127.
doi: 10.3184/030823404323001052 |
| [64] |
Li, J. S.; Wang, Y. G.; Sun, L. G.; Xu, X. H. Mater. Prot. 2010, 43, 28 (in Chinese).
|
|
(李久盛, 王永刚, 孙令国, 徐小红, 材料保护, 2010, 43, 28.)
|
|
| [65] |
Zhang, B. X.; Wu, Q.; Chao, J. B.; Guo, Y. L. Chin. J. Synth. Chem. 2013, 21, 86 (in Chinese).
|
|
(张变香, 吴群, 钞建宾, 郭一力, 合成化学, 2013, 21, 86.)
|
|
| [66] |
Zhang, B.; Kang, Y.; Yang, L.; Chen, X. ChemistrySelect 2016, 1, 1529.
doi: 10.1002/slct.v1.8 |
| [67] |
Bhujabal, Y. B.; Vadagaonkar, K. S.; Gholap, A.; Sanghvi, Y. S.; Dandela, R.; Kapdi, A. R. J. Org. Chem. 2019, 84, 15343.
doi: 10.1021/acs.joc.9b02371 |
| [68] |
Dumas, J.; Brittelli, D.; Chen, J. S.; Dixon, B.; Hatoum-Mokdad, H.; Konig, G.; Sibley, R.; Witowsky, J.; Wong, S. Bioorg. Med. Chem. Lett. 1999, 9, 2531.
doi: 10.1016/S0960-894X(99)00433-3 |
| [69] |
Mavrova, A. T.; Anichina, K. K.; Vuchev, D. I.; Tsenov, J. A.; Denkova, P. S.; Kondeva, M. S.; Micheva, M. K. Eur. J. Med. Chem. 2006, 41, 1412.
doi: 10.1016/j.ejmech.2006.07.005 |
| [70] |
Zhao, P. L.; Wang, F.; Huang, W.; Chen, Q.; Liu, Z. M. Chin. J. Org. Chem. 2010, 30, 1567 (in Chinese).
|
|
(赵培亮, 王福, 黄伟, 陈琼, 刘祖明, 有机化学, 2010, 30, 1567.)
|
|
| [71] |
Maske, P.; Lokapure, S.; Nimbalkar, D.; Disouza, J. Pharma Chem. 2012, 4, 1283.
|
| [72] |
Perez-Villanueva, J.; Hernandez-Campos, A.; Yepez-Mulia, L.; Mendez-Cuesta, C.; Mendez-Lucio, O.; Hernandez-Luis, F.; Castillo, R. Bioorg. Med. Chem. Lett. 2013, 23, 4221.
doi: 10.1016/j.bmcl.2013.05.012 |
| [73] |
Yoon, H. J.; Yang, S. J.; Gong, Y. D. ACS Comb. Sci. 2017, 19, 738.
doi: 10.1021/acscombsci.7b00106 |
| [74] |
Song, M. X.; Huang, Y.; Wang, S.; Wang, Z. T.; Deng, X. Q. Arch. Pharm. Chem. Life Sci. 2019, 352, 1800313.
doi: 10.1002/ardp.v352.8 |
| [75] |
Liu, Z.; Bian, M.; Ma, Q. Q.; Zhang, Z.; Du, H. H.; Wei, C. X. Molecules 2020, 25, 5391.
doi: 10.3390/molecules25225391 |
| [1] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [2] | Jiaxia Pu, Xiaoying Jia, Lirong Han, Qinghan Li. Research Progress of Visible Light Promoted C—N Bond Fracture to Construct C—C Bond [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2591-2613. |
| [3] | Min Wu, Bo Liu, Jialong Yuan, Qiang Fu, Rui Wang, Dawei Lou, Fushun Liang. Recent Progress in the C—S Bond Formation Reactions Mediated by Visible Light [J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2269-2292. |
| [4] | Zujia Lu, Jian Qin, Jinting Wu, Wenli Cao, Baolong Kuang, Jianguo Zhang. Advances in the Synthesis of Energetic Compounds Based on 1,2,3-Triazoles [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 526-554. |
| [5] | Peng Liu, Fuming Zhong, Lihao Liao, Weiqiang Tan, Xiaodan Zhao. Progress in the Construction of Spirocyclohexadienones via Alkyne-Involving Dearomatization [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4019-4035. |
| [6] | Fei Cheng, Qiwen Sun, Jiangrong Lu, Xinglan Wang, Jiquan Zhang. Research Progress on the Construction of C—S Bond Using Aryl Disulfides as Radical Sulfur Reagents [J]. Chinese Journal of Organic Chemistry, 2023, 43(11): 3728-3744. |
| [7] | Can Yong, Yun Li, Tao Bi, Guofeng Chen, Dongxia Zheng, Zhouyu Wang, Yuanyuan Zhang. Research Progress on the Synthesis and Activity of D-Galactose Derived Small Galectin Inhibitors [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1307-1325. |
| [8] | Zhihao Zhang, Xin Jiang, Qinghan Li. Recent Progress in the Synthesis of Substituted Benzo[b]furan Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 945-964. |
| [9] | Changxing Ji, Guangxia Wang, Hua Wang. Progress in Metal-Organic Supramolecular System Based on Subcomponent Self-Assembly [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2261-2279. |
| [10] | Yuting Liu, Jie Li, Dawei Yin. Progress of Ferrocene-Based Metal Cation Recognition Receptor [J]. Chinese Journal of Organic Chemistry, 2021, 41(1): 158-170. |
| [11] | Wang Shoufeng, Wang Wengui. Recent Advances of the Construction of Trifluoromethylated Quaternary Carbon Center [J]. Chinese Journal of Organic Chemistry, 2020, 40(7): 1901-1911. |
| [12] | Jia Qianfa, Li Yaqiong, Lin Yinhe. Recent Advances in Organocatalyzed Aromatization Reactions [J]. Chinese Journal of Organic Chemistry, 2020, 40(6): 1502-1513. |
| [13] | Zhang Yanxia, Han Jianwei. Recent Progress of Chiral Iminophosphorane Catalysis [J]. Chinese Journal of Organic Chemistry, 2020, 40(10): 3154-3165. |
| [14] | Li Weilin, Chen Xuanying, Zheng Tianjiao, Zou Qi, Chen Wenbo. Research Progress on Reduction of Sulfoxides to Thiothers [J]. Chin. J. Org. Chem., 2019, 39(9): 2443-2457. |
| [15] | Yang Yang, Guo Ju, Liu Zhanzhu. Progress in the Synthesis of Analogues of Bistetrahdro-isoquinoline Antitumor Alkaloids [J]. Chin. J. Org. Chem., 2019, 39(7): 1913-1922. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||