Chinese Journal of Organic Chemistry ›› 2021, Vol. 41 ›› Issue (11): 4428-4436.DOI: 10.6023/cjoc202106015 Previous Articles Next Articles
ARTICLES
韩立卓a, 赵丽敏a, 王慧敏a, 窦桐a, 郭芳妍a, 齐浚答a, 许文博a, 朴莲荀a, 金学军a, 陈芬儿b, 朴虎日b, 郑昌吉b,*( ), 金成华a,b,*(
), 金成华a,b,*( )
)
收稿日期:2021-06-07
修回日期:2021-07-12
发布日期:2021-08-09
通讯作者:
郑昌吉, 金成华
作者简介:基金资助:
Lizhuo Hana, Limin Zhaoa, Huimin Wanga, Tong Doua, Fangyan Guoa, Junda Qia, Wenbo Xua, Lianxun Piaoa, Xuejun Jina, Fen'er Chenb, Huri Piaob, Changji Zhengb( ), Chenghua Jina,b(
), Chenghua Jina,b( )
)
Received:2021-06-07
Revised:2021-07-12
Published:2021-08-09
Contact:
Changji Zheng, Chenghua Jin
About author:Supported by:Share
Lizhuo Han, Limin Zhao, Huimin Wang, Tong Dou, Fangyan Guo, Junda Qi, Wenbo Xu, Lianxun Piao, Xuejun Jin, Fen'er Chen, Huri Piao, Changji Zheng, Chenghua Jin. Synthesis, Antibacterial and Antifungal Evaluation of Rhodanine Derivatives Bearing Quinoxalinyl Imidazole Moiety as ALK5 Inhibitors[J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4428-4436.
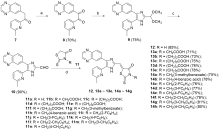

| Compd. | R | IC50a/(μmol•L–1) | Selectivity indexb | |
|---|---|---|---|---|
| p38α | ALK5 | |||
| 12 | H | >10 | 3.215 | >3 |
| 13a | CH2COOH | 4.417 | 1.027 | 4 |
| 13b | (CH2)2COOH | >10 | 1.137 | >8 |
| 13c | (CH2)3COOH | >10 | 0.614 | >16 |
| 13d | (CH2)4COOH | >10 | 2.276 | >4 |
| 13e | (CH2)5COOH | >10 | 0.451 | >22 |
| 14a | CH2(3-methyl- benzoate) | >10 | 1.783 | >5 |
| 14b | CH2(4-benzoic acid) | >10 | 0.500 | >20 |
| 14c | CH2(2-FC6H4) | >10 | 1.709 | >5 |
| 14d | CH2(3-FC6H4) | >10 | 0.954 | >10 |
| 14e | CH2(4-FC6H4) | >10 | 3.037 | >3 |
| 14f | CH2(2-CH3C6H4) | >10 | 0.494 | >20 |
| 14g | CH2(3-CH3C6H4) | >10 | 5.076 | >1 |
| 14h | CH2(4-CH3C6H4) | >10 | 0.919 | >10 |
| 2 (LY-2157299) | 0.493 | 0.129 | 4 | |
| Compd. | R | IC50a/(μmol•L–1) | Selectivity indexb | |
|---|---|---|---|---|
| p38α | ALK5 | |||
| 12 | H | >10 | 3.215 | >3 |
| 13a | CH2COOH | 4.417 | 1.027 | 4 |
| 13b | (CH2)2COOH | >10 | 1.137 | >8 |
| 13c | (CH2)3COOH | >10 | 0.614 | >16 |
| 13d | (CH2)4COOH | >10 | 2.276 | >4 |
| 13e | (CH2)5COOH | >10 | 0.451 | >22 |
| 14a | CH2(3-methyl- benzoate) | >10 | 1.783 | >5 |
| 14b | CH2(4-benzoic acid) | >10 | 0.500 | >20 |
| 14c | CH2(2-FC6H4) | >10 | 1.709 | >5 |
| 14d | CH2(3-FC6H4) | >10 | 0.954 | >10 |
| 14e | CH2(4-FC6H4) | >10 | 3.037 | >3 |
| 14f | CH2(2-CH3C6H4) | >10 | 0.494 | >20 |
| 14g | CH2(3-CH3C6H4) | >10 | 5.076 | >1 |
| 14h | CH2(4-CH3C6H4) | >10 | 0.919 | >10 |
| 2 (LY-2157299) | 0.493 | 0.129 | 4 | |
| Compd. | R | Gram-positive strains | Gram-negative strains | Fungus 7535e | |||
|---|---|---|---|---|---|---|---|
| 4220a | 209b | 1924c | 2742d | ||||
| 12 | H | >64 | >64 | >64 | >64 | 0.5 | |
| 13a | CH2COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13b | (CH2)2COOH | >64 | >64 | >64 | 64 | 1 | |
| 13c | (CH2)3COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13d | (CH2)4COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13e | (CH2)5COOH | >64 | >64 | >64 | >64 | >64 | |
| 14a | CH2(3-methyl- benzoate) | >64 | >64 | >64 | >64 | 0.5 | |
| 14b | CH2(4-benzoic acid) | >64 | >64 | >64 | >64 | 0.5 | |
| 14c | CH2(2-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14d | CH2(3-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14e | CH2(4-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14f | CH2(2-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14g | CH2(3-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14h | CH2(4-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| Gatifloxacin | 0.25 | 1 | 2 | 1 | 0.5 | ||
| Fluconazole | N.Df | N.D | N.D | N.D | 1 | ||
| Compd. | R | Gram-positive strains | Gram-negative strains | Fungus 7535e | |||
|---|---|---|---|---|---|---|---|
| 4220a | 209b | 1924c | 2742d | ||||
| 12 | H | >64 | >64 | >64 | >64 | 0.5 | |
| 13a | CH2COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13b | (CH2)2COOH | >64 | >64 | >64 | 64 | 1 | |
| 13c | (CH2)3COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13d | (CH2)4COOH | >64 | >64 | >64 | >64 | 0.5 | |
| 13e | (CH2)5COOH | >64 | >64 | >64 | >64 | >64 | |
| 14a | CH2(3-methyl- benzoate) | >64 | >64 | >64 | >64 | 0.5 | |
| 14b | CH2(4-benzoic acid) | >64 | >64 | >64 | >64 | 0.5 | |
| 14c | CH2(2-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14d | CH2(3-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14e | CH2(4-FC6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14f | CH2(2-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14g | CH2(3-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| 14h | CH2(4-CH3C6H4) | >64 | >64 | >64 | >64 | 0.5 | |
| Gatifloxacin | 0.25 | 1 | 2 | 1 | 0.5 | ||
| Fluconazole | N.Df | N.D | N.D | N.D | 1 | ||
| Compd. | MIC (µmol•L–1)a | IC50b,c/ (µmol•L–1) | Compd. | MIC/ (µmol•L–1) | IC50/ (µmol•L–1) |
|---|---|---|---|---|---|
| 12 | 1.16 | 39.86 | 14b | 0.89 | 58.27 |
| 13a | 1.02 | 43.26 | 14c | 0.93 | 53.24 |
| 13b | 1.99 | 44.28 | 14d | 0.93 | 54.96 |
| 13c | 0.97 | 46.20 | 14e | 0.93 | 54.38 |
| 13d | 0.94 | 47.36 | 14f | 0.94 | 55.29 |
| 13e | >117.5 | 48.02 | 14g | 0.94 | 51.84 |
| 14a | 0.86 | 52.86 | 14h | 0.94 | 53.25 |
| Compd. | MIC (µmol•L–1)a | IC50b,c/ (µmol•L–1) | Compd. | MIC/ (µmol•L–1) | IC50/ (µmol•L–1) |
|---|---|---|---|---|---|
| 12 | 1.16 | 39.86 | 14b | 0.89 | 58.27 |
| 13a | 1.02 | 43.26 | 14c | 0.93 | 53.24 |
| 13b | 1.99 | 44.28 | 14d | 0.93 | 54.96 |
| 13c | 0.97 | 46.20 | 14e | 0.93 | 54.38 |
| 13d | 0.94 | 47.36 | 14f | 0.94 | 55.29 |
| 13e | >117.5 | 48.02 | 14g | 0.94 | 51.84 |
| 14a | 0.86 | 52.86 | 14h | 0.94 | 53.25 |
| [1] |
Jin, C. H.; Krishnaiah, M.; Sreenu, D.; Rao, K. S.; Subrahmanyam, V. B.; Park, C. Y.; Son, J. Y.; Sheen, Y. Y.; Kim, D. K. Bioorg. Med. Chem. 2011, 19, 2633.
doi: 10.1016/j.bmc.2011.03.008 |
| [2] |
Ma, J.; Mi, C.; Wang, K. S.; Lee, J. J.; Jin, X. J. Pharmacol. Sci. 2016, 130, 43.
doi: 10.1016/j.jphs.2015.10.002 |
| [3] |
Zhang, Z. H.; Mi, C.; Wang, K. S.; Wang, Z.; Li, M. Y.; Zuo, H. X.; Xu, G. H.; Li, X.; Piao, L. X.; Ma, J.; Jin, X. Phytother. Res. 2018, 32, 65.
doi: 10.1002/ptr.5948 pmid: 29044876 |
| [4] |
Shang, Y.; Han, X.; Yao, Y. L.; Li, Y. M.; Zhang, J.; Shao, D. Y.; Hou, L. S.; Fan, Y.; Song, S. Z.; Lian, L. H.; Nan, J. X.; Wu, Y. L. Biomed. Pharmacother. 2018, 107, 374.
doi: S0753-3322(18)34029-0 pmid: 30099341 |
| [5] |
Lu, Y.; Xu, X.; Jiang, T.; Jin, L.; Zhao, X. D.; Cheng, J. H.; Jin, X. J.; Ma, J. Piao, H. N.; Piao, L. X. Int. Immunopharmacol. 2019, 67, 119.
doi: 10.1016/j.intimp.2018.12.011 |
| [6] |
Xing, Y.; Mi, C.; Wang, Z.; Zhang, Z. H.; Li, M.; Zuo, H. X.; Wang, J. Y.; Jin, X.; Ma, J. Pharmacol. Res. 2018, 135, 166.
doi: S1043-6618(18)30660-1 pmid: 30103001 |
| [7] |
Wang, Z.; Li, M. Y.; Zhang, Z. H.; Zuo, H. X.; Wang, J. Y.; Xing, Y.; Ri, M. H.; Jin, H. L.; Jin, C. H.; Xu, G. H.; Piao, L. X.; Jiang, C. G.; Ma, J.; Jin, X. Pharmacol. Res. 2020, 155, 104727.
doi: S1043-6618(19)32925-1 pmid: 32113874 |
| [8] |
Cao, Y. P.; Pan, M.; Song, Y. L.; Zhang, H. L.; Su, H. T.; Shan, B. C.; Piao, H. X. Eur. Rev. Med. Pharmacol. 2019, 23, 7863.
|
| [9] |
Chen, S.; Zhang, Q.; Xu, D.; Li, Y.; Fan, Y.; Li, W.; Yin, X.; Zhang, Y.; Liu, J.; Li, X.; Sun, L.; Jin, N. Anti-cancer Drug 2018, 29, 197.
|
| [10] |
Wu, Y. L.; Zhang, Y. J.; Yao, Y. L.; Li, Z. M.; Han, X.; Lian, L. H.; Zhao, Y. Q.; Nan, J. X. Toxicol. Lett. 2016, 258, 147.
doi: 10.1016/j.toxlet.2016.06.2102 |
| [11] |
Zhu, W. J.; Cui, B. W.; Wang, H. M.; Nan, J. X.; Piao, H. R.; Lian, L. H.; Jin, C. H. Eur. J. Med. Chem. 2019, 180, 15.
doi: 10.1016/j.ejmech.2019.07.013 |
| [12] |
Guo, Z.; Song, X.; Zhao, L. M.; Piao, M. G.; Quan, J.; Piao, H. R.; Jin, C. H. Bioorg. Med. Chem. Lett. 2019, 29, 2070.
doi: 10.1016/j.bmcl.2019.07.015 |
| [13] |
Li, Y. W.; Li, X. Y.; Li, S.; Zhao, L. M.; Ma, J.; Piao, H. R.; Jiang, Z.; Jin, C. H.; Jin, X. Bioorg. Med. Chem. Lett. 2020, 30, 126822.
doi: 10.1016/j.bmcl.2019.126822 |
| [14] |
Derynck, R.; Zhang, Y. E. Nature 2003, 425, 577.
doi: 10.1038/nature02006 |
| [15] |
Jin, C. H.; Krishanaiah, M.; Sreenu, D.; Subrahmanyam, V. B.; Park, H. J.; Park, S. J.; Sheen, Y. Y.; Kim, D. K. Bioorg. Med. Chem. 2014, 22, 2724.
doi: 10.1016/j.bmc.2014.03.022 |
| [16] |
Brandes, A. A.; Carpentier, A. F.; Kesari, S.; Sepulveda-Sanchez, J. M.; Wheeler, H. R.; Chinot, O.; Cher, L.; Steinbach, J. P.; Capper, D.; Specenier, P.; Rodon, J.; Cleverly, A.; Smith, C.; Gueorguieva, I.; Miles, C.; Guba, S. C.; Desaiah, D.; Lahn, M. M.; Wick, W. A. Neuro. Oncol. 2016, 18, 1146.
doi: 10.1093/neuonc/now009 |
| [17] |
Battle, E.; Massague, J. Immunity 2019, 50, 924.
doi: 10.1016/j.immuni.2019.03.024 |
| [18] |
Jin, C. H.; Krishnaiah, M.; Sreenu, D.; Subrahmanyam, V. B.; Rao, K. S.; Lee, H. J.; Park, S. J.; Park, H. J.; Lee, K.; Sheen, Y. Y.; Kim, D. K. J. Med. Chem. 2014, 57, 4213.
doi: 10.1021/jm500115w |
| [19] |
Jin, C. H.; Sreenu, D.; Krishnaiah, M.; Subrahmanyam, V. B.; Rao, K. S.; Mohan, A. V. N.; Park, C. V.; Son, J. Y.; Son, D. H.; Park, H. J.; Sheen, Y. Y.; Kim, D. K. Eur. J. Med. Chem. 2011, 46, 3917.
doi: 10.1016/j.ejmech.2011.05.063 |
| [20] |
Zhao, L. M.; Guo, Z.; Xue, Y. J.; Min, J. Z.; Zhu, W. J.; Li, X. Y.; Piao, H. R.; Jin, C. H. Molecules 2018, 23, 3369.
doi: 10.3390/molecules23123369 |
| [21] |
Patel, H. M.; Sing, B.; Bhardwaj, V.; Palkar, M.; Shaikh, M. S.; Rane, R.; Alwan, W. S.; Gadad, A. K.; Noolvi, M. N.; Karpoormath, R. Eur. J. Med. Chem. 2015, 93, 599.
doi: 10.1016/j.ejmech.2014.09.002 |
| [22] |
Devi, P. B.; Samala, G.; Sridevi, J. P.; Saxena, S.; Alvala, M.; Salina, E. G.; Sriam, D.; Yogeeswari, P. Chem. Med. Chem. 2014, 9, 2538.
doi: 10.1002/cmdc.201402171 |
| [23] |
Ottanà, R.; Paoli, P.; Naß, A.; Lori, G.; Gardile, V.; Adornato, I.; Rotondo, A.; Graziano, A. C. E.; Wolber, G.; Maccari, R. Eur. J. Med. Chem. 2017, 127, 840.
doi: 10.1016/j.ejmech.2016.10.063 |
| [24] |
Liang, Y.; Tang, M. L.; Huo, Z.; Zhang, C.; Sun, X. Molecules 2020, 25, 1138.
doi: 10.3390/molecules25051138 |
| [25] |
Chen, Z. H.; Zheng, C. J.; Sun, L. P.; Piao, H. R. Eur. J. Med. Chem. 2010, 45, 5739.
doi: 10.1016/j.ejmech.2010.09.031 pmid: 20889240 |
| [26] |
Khodair, A. I.; Awad, M. K.; Gesson, J. P.; Elshaier, Y. A. M. M. Carbohydr. Res. 2020, 487, 107894.
doi: 10.1016/j.carres.2019.107894 |
| [27] |
Russell, A. J.; Westwood, I. M.; Crawford, M. H. J.; Robinson, J.; Kawamura, A.; Redfield, C.; Laurieri, N.; Lowe, E. D.; Davies, S. G.; Sim, E. Bioorg. Med. Chem. 2009, 17, 905.
doi: 10.1016/j.bmc.2008.11.032 |
| [28] |
Gandini, A.; Bartolini, M.; Tedesco, D.; Martinez-Gonzalez, L.; Roca, C.; Campillo, N. E.; Zaldivar-Diez, J.; Perez, C.; Zuccheri, G.; Miti, A.; Feoli, A.; Castellano, S.; Petralla, S.; Petralla, S.; Monti, B.; Rossi, M.; Moda, F.; Legname, G.; Martinez, A.; Bolognesi, M. L. J. Med. Chem. 2018, 61, 7640.
doi: 10.1021/acs.jmedchem.8b00610 |
| [29] |
Song, M. X.; Zheng, C. J.; Deng, X. Q.; Wang, Q.; Hou, S. P.; Liu, T. T.; Xing, X. L.; Piao, H. R. Eur. J. Med. Chem. 2012, 54, 403.
doi: 10.1016/j.ejmech.2012.05.023 |
| [30] |
Guo, M.; Zheng, C. J.; Song, M. X.; Wu, Y.; Sun, L. P.; Li, Y. J.; Liu, Y.; Piao, H. R. Bioorg. Med. Chem. Lett. 2013, 23, 4358.
doi: 10.1016/j.bmcl.2013.05.082 |
| [31] |
Song, M. X.; Zheng, C. J.; Deng, X. Q.; Sun, L. P.; Wu, Y.; Hong, L.; Li, Y. J.; Liu, Y.; Wei, Z. Y.; Jin, M. J.; Piao, H. R. Eur. J. Med. Chem. 2013, 60, 376.
doi: 10.1016/j.ejmech.2012.12.007 |
| [32] |
Wei, Z. Y.; Liu, J. C.; Zhang, W.; Li, Y. R.; Li, C.; Zheng, C. J.; Piao, H. R. Med. Chem. 2016, 12, 751.
doi: 10.2174/1573406412666160822160156 |
| [33] |
Liu, X. F.; Zheng, C. J.; Sun, L. P.; Liu, X. K.; Piao, H. R. Eur. J. Med. Chem. 2011, 46, 3469.
doi: 10.1016/j.ejmech.2011.05.012 |
| [34] |
Hardej, D.; Ashby, Jr C. R.; Khadtare, N. S.; Kulkarni, S. S.; Singh, S.; Talele, T. T.; Eur. J. Med. Chem. 2010, 45, 5827.
doi: 10.1016/j.ejmech.2010.09.045 pmid: 20947220 |
| [35] |
Zhang, T. Y.; Li, C.; Tian, Y. S.; Li, J. J.; Sun, L. P.; Zheng, C. J.; Piao, H. R. Chin. Chem. Lett. 2017, 58, 1737.
|
| [36] |
Zuo, H. X.; Jin, Y.; Wang, Z.; Li, M. Y.; Zhang, Z. H.; Wang, J. Y.; Xing, Y.; Ri, M. H.; Jin, C. H.; Xu, G. H.; Piao, L. X.; Ma, J.; Jin, X. J. Ethnopharmacol. 2020, 257, 112835.
doi: 10.1016/j.jep.2020.112835 |
| [37] |
Xu, X.; Jin, L.; Jiang, T.; Lu, Y.; Aosai, F.; Piao, H. N.; Xu, G. H.; Jin, C. H.; Jin, X. J.; M. J.; Piao, L. X.; J. Ginseng Res. 2020, 44, 704.
doi: 10.1016/j.jgr.2019.06.002 |
| [38] |
Kim, D. K.; Jung, S. H.; Lee, H. S.; Dewang, P. M. Eur. J. Med. Chem. 2009, 44, 568.
doi: 10.1016/j.ejmech.2008.03.024 |
| [39] |
Kim, D. K.; Jang, Y.; Lee, H. S.; Park, H. J.; Yoo, J. J. Med. Chem. 2007, 50, 3143.
doi: 10.1021/jm070129k |
| [40] |
Xu, G.; Zhang, Y.; Wang, H.; Guo, Z.; Wang, X.; Li, X.; Chang, S.; Sun, T.; Yu, Z.; Xu, T.; Zhao, L.; Wang, Y.; Yu, W. Eur. J. Med. Chem. 2020, 198, 112354.
doi: 10.1016/j.ejmech.2020.112354 |
| [1] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [2] | Mingxia Song, Yangnv Zhu, Shishuai Wang, Yuping Huang, Xianqing Deng, Yushan Huang. Synthesis and Antibacterial Activity Evaluation of Guanidine Hydrazone Derivatives Containing Linear Alkanes [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2163-2170. |
| [3] | Meng Li, Dongguo Xia, Yunxiao Wang, Xiang Cheng, Jiexiu Gong, Yao Chen, Xianhai Lü. Design, Synthesis and Antifungal Bioactivity Evaluation of Thiazole Benzoate Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 686-696. |
| [4] | Wei Chen, Simin Lei, Yuxin Lan, Haojian Xu, Pingbin Yu, Rui Zhang, Run Wu, Yang Chen. Design, Synthesis and Antifungal Activities of Novel Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2164-2171. |
| [5] | Xiu Wang, Wengui Duan, Guishan Lin, Baoyu Li, Wenjing Zhang, Fuhou Lei. Synthesis, Antifungal Activity, Three-Dimensional Quantitative Structure-Activity Relationship and Molecular Docking Study of 4-Acyl-3-amino-1,2,4-triazole-thioether Derivatives Containing Natural Pinene Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 871-883. |
| [6] | Yan Zeng, Lifei Nie, Chao Niu, Aytilla Mamatjan, Khurshed Bozorov, Jiangyu Zhao, Haji Akber Aisa. Synthesis and Biological Activities of Dihydrooxazolo[5,4-d]-pyrrolo[1,2-a]pyrimidinones [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 543-556. |
| [7] | Wei Wang, Furan Wu, Yidan Ma, Dan Xu, Gong Xu. Study on Synthesis and Antifungal Activity of Novel Benzamides Containing Substituted Pyrazole Unit [J]. Chinese Journal of Organic Chemistry, 2022, 42(2): 607-618. |
| [8] | Yucheng Cui, Meihua Chen, Guishan Lin, Wengui Duan, Qingmin Li, Renxuan Zou, Bo Cen. Synthesis, Antifungal Activity and Molecular Docking Study of 1,3,4-Thiadiazole-Urea Compounds Containing gem-Dimethylcyclopropane Ring Structure [J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3784-3797. |
| [9] | Jinjiao Dong, Xinyue Zhu, Siran Feng, Chaochao Zhang, Zhenming Liu, Xiaoqiang Qiao, Yali Song. Synthesis and Antifungal Activity of 7-Phenyl-6H,7H-1,3,4-thia- diazolo[3,2-a]-thiochromeno[4,3-d]pyrimidine Compounds [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2467-2475. |
| [10] | Yujia Sun, Ziwei Wang, Yan Wang, Tongxiu Xu, Keqing Tian, Ping Zhang. Synthesis and Antimicrobial Activity of 1,5-Benzothiazepines Incorporated with Triazole Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2361-2373. |
| [11] | Weiwei Wang, Yu Zhao, Xinlei Liu, Jiazhen Jiang, Ming'an Wang. Synthesis and Antifungal Activity of 3-Aryl-7-methyl- 7-hydroxy-2-octen-6-olide [J]. Chinese Journal of Organic Chemistry, 2021, 41(6): 2343-2353. |
| [12] | Hongbo Dong, Weiwei Wang, Yu Zhao, Xinlei Liu, Ming'an Wang. Synthesis and Antifungal Activity of 3,7-Dimethyl-7-hydroxy-2-octen-6-olide Analogues [J]. Chinese Journal of Organic Chemistry, 2021, 41(4): 1646-1657. |
| [13] | Xibin Wu, Yinfeng Tan, Jiling Yi, Xinming Song, Jingyu Yang, Xueming Zhou, Guangying Chen. Study on Bioactive Secondary Metabolites from Penicillium herquei JX4 [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 1251-1254. |
| [14] | Jun Shi, Na Luo, Muhan Ding, Chuanhui Li, Suran Wan, Peijia Li, Junhong Li, Xiaoping Bao. Synthesis and Antimicrobial Activities of Novel 1,3,4-Oxa(Thia)- diazole Derivatives Containing 6-Fluoroquinazoline Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(2): 738-756. |
| [15] | Li Anbang, Li Zhongshan, Zhao Yang, Yao Tingting, Cheng Jingli, Zhao Jinhao. Design, Synthesis and Antifungal Activity of Novel Pyrazole-Thiophene Carboxamide Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(9): 2836-2844. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||