Chinese Journal of Organic Chemistry ›› 2023, Vol. 43 ›› Issue (1): 223-228.DOI: 10.6023/cjoc202206038 Previous Articles Next Articles
ARTICLES
收稿日期:2022-06-21
修回日期:2022-07-26
发布日期:2022-09-01
通讯作者:
王静
基金资助:
Jing Wang( ), Linlin Wu, Qian Wang
), Linlin Wu, Qian Wang
Received:2022-06-21
Revised:2022-07-26
Published:2022-09-01
Contact:
Jing Wang
Supported by:Share
Jing Wang, Linlin Wu, Qian Wang. Synthesis and Characterization of New Indeno[1,2-b]fluorene-6,12-dione Derivatives[J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 223-228.
| Compound | λabs/nm | Ega/eV | λem/nm | Tdec/℃ | Eox/V | Eonset ox/V | E1 red/V | E2 red/V | Eonset red/V | EHOMOb/eV | ELUMOc/eV | Eel g/eV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
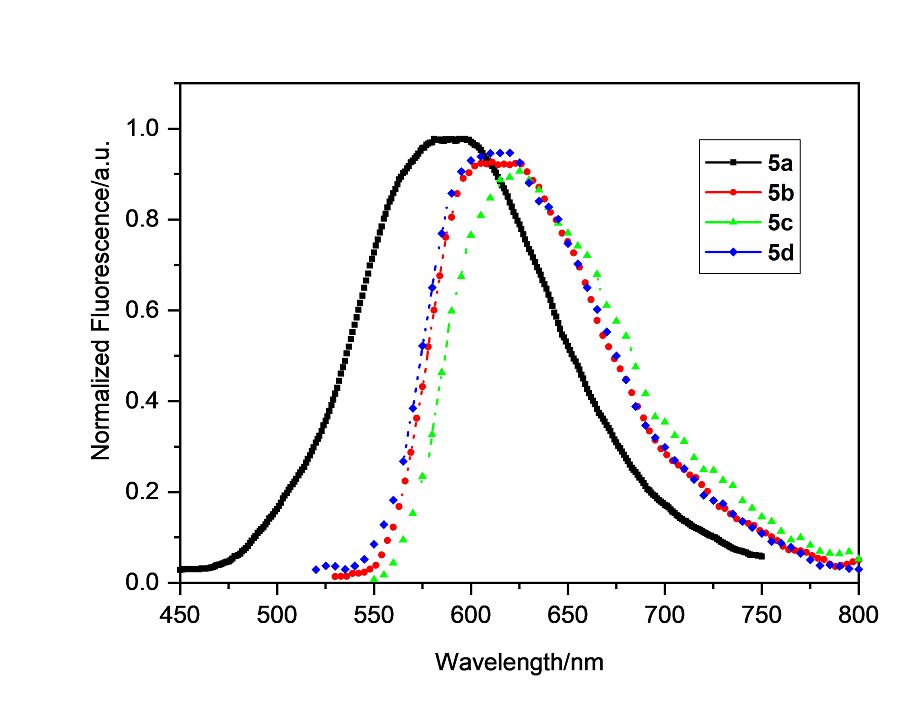
| 5a | 495, 315, 293 | 2.25 | 590 | 390 | ||||||||
| 5b | 508, 335, 295 | 2.12 | 610 | 345 | 1.69 | 1.51 | –1.13 | –1.54 | –0.98 | –5.91 | –3.42 | 2.49 |
| 5c | 509, 335, 295 | 2.13 | 625 | 385 | 1.70 | 1.53 | –1.12 | –1.53 | –0.95 | –5.93 | –3.45 | 2.48 |
| 5d | 505, 335, 295 | 2.14 | 617 | 400 | 1.69 | 1.52 | –1.13 | –1.54 | –0.98 | –5.92 | –3.42 | 2.50 |
| Compound | λabs/nm | Ega/eV | λem/nm | Tdec/℃ | Eox/V | Eonset ox/V | E1 red/V | E2 red/V | Eonset red/V | EHOMOb/eV | ELUMOc/eV | Eel g/eV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 495, 315, 293 | 2.25 | 590 | 390 | ||||||||
| 5b | 508, 335, 295 | 2.12 | 610 | 345 | 1.69 | 1.51 | –1.13 | –1.54 | –0.98 | –5.91 | –3.42 | 2.49 |
| 5c | 509, 335, 295 | 2.13 | 625 | 385 | 1.70 | 1.53 | –1.12 | –1.53 | –0.95 | –5.93 | –3.45 | 2.48 |
| 5d | 505, 335, 295 | 2.14 | 617 | 400 | 1.69 | 1.52 | –1.13 | –1.54 | –0.98 | –5.92 | –3.42 | 2.50 |
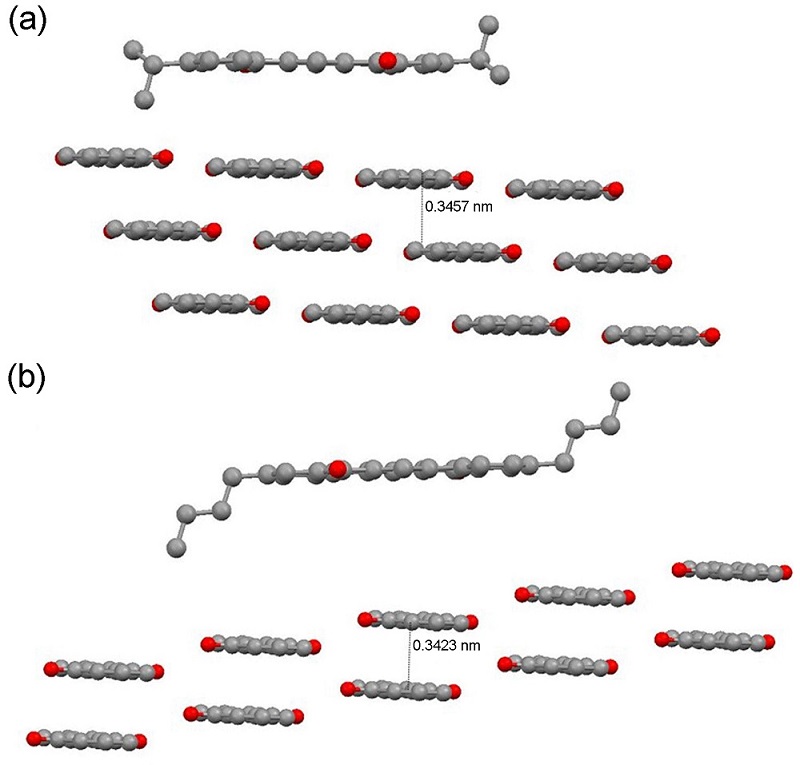
| [1] |
Jiang, H.; Zhu, S. L.; Cui, Z. D.; Li, Z. Y.; Liang, Y. Q.; Zhu, J. M.; Hu, Peng.; Zhang, H. L.; Hu, W. P. Chem. Soc. Rev. 2022, 51, 3071.
doi: 10.1039/D1CS01136G |
| [2] |
Jiang, H.; Hu, W. P. Angew. Chem., Int. Ed. 2020, 59, 1408.
doi: 10.1002/anie.201814439 pmid: 30927312 |
| [3] |
Dong, H. L.; Zhu, H. F.; Meng, Q.; Gong, X.; Hu, W. P. Chem. Soc. Rev. 2012, 41, 1754.
doi: 10.1039/C1CS15205J |
| [4] |
Baeg, K. J.; Caironi, M.; Noh, Y. Y. Adv. Mater. 2013, 25, 4210.
doi: 10.1002/adma.201205361 |
| [5] |
Koo, J. H.; Kim, D. C.; Shim, H. J.; Kim, T. H.; Kim, D. H. Adv. Funct. Mater. 2018, 28, 1801834.
|
| [6] |
Zschieschang, U.; Yamamoto, T.; Takimiya, K.; Kuwabara, H.; Ikeda, M.; Sekitani, T.; Someya, T.; Klauk, H. Adv. Mater. 2011, 23, 654.
doi: 10.1002/adma.201003374 |
| [7] |
Zhan, Y. Q.; Mei, Y. F.; Zheng, L. R. J. Mater. Chem. C 2014, 2, 1220.
doi: 10.1039/C3TC31765J |
| [8] |
Zhang, C. C.; Chen, P. L.; Hu, W. P. Chem. Soc. Rev. 2015, 44, 2087.
doi: 10.1039/C4CS00326H |
| [9] |
Chen, D.; Pei, Q. B. Chem. Rev. 2017, 117, 11239.
doi: 10.1021/acs.chemrev.7b00019 |
| [10] |
Han, S. T.; Zhou, Y.; Roy, V. A. L. Adv. Mater. 2013, 25, 5425.
doi: 10.1002/adma.201301361 |
| [11] |
McNellis, E. R.; Schott, S.; Sirringhaus, H.; Sinova, J. Phys. Rev. Mater. 2018, 2, 074405.
|
| [12] |
Kuehne, A. J. C.; Gather, M. C. Chem. Rev. 2016, 116, 12823.
doi: 10.1021/acs.chemrev.6b00172 |
| [13] |
Liu, Y.; Yu, G.; Liu, Y. Q. Sci. China: Chem. 2010, 53, 779.
doi: 10.1007/s11425-010-0040-8 |
| [14] |
Cai, Z. X.; Awais, M. A.; Zhang, N.; Yu, L. P. Chem, 2018, 4, 2538.
doi: 10.1016/j.chempr.2018.08.017 |
| [15] |
Cinar, M. E.; Ozturk, T. Chem. Rev. 2015, 115, 3036.
doi: 10.1021/cr500271a |
| [16] |
Dai, X. X.; Cheng, X. D.; Kan, Z. P.; Xiao, Z. Y.; Duan, T. N.; Hu, C.; Lu, S. R. Chin. J. Org. Chem. 2020, 40, 4031. (in Chinese)
doi: 10.6023/cjoc202005023 |
|
(戴学新, 成晓东, 阚志鹏, 肖泽云, 段泰男, 胡超, 陆仕荣, 有机化学, 2020, 40, 4031.)
|
|
| [17] |
Li, H. Y.; Tee, B. C. K.; Cha, J. J.; Cui, Y.; Chung, J. W.; Lee, S. Y.; Bao, Z. N. J. Am. Chem. Soc. 2012, 134, 2760.
doi: 10.1021/ja210430b |
| [18] |
Dong, H. L.; Hu, W. P. Acc. Chem. Res. 2016, 49, 2435.
doi: 10.1021/acs.accounts.6b00368 |
| [19] |
Kitamura, M.; Arakawa, Y. J. Phys.: Condens. Matter 2008, 20, 184011.
doi: 10.1088/0953-8984/20/18/184011 |
| [20] |
Jurchescu, O. D.; Popinciuc, M.; van Wees, B. J.; Palstra, T. T. M. Adv. Mater. 2007, 19, 688.
doi: 10.1002/adma.200600929 |
| [21] |
Maliakal, A.; Raghavachari, K.; Katz, H.; Chandross, E.; Siegrist, T. Chem. Mater. 2004, 16, 4980.
doi: 10.1021/cm049060k |
| [22] |
Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. J. Am. Chem. Soc. 2007, 129, 15732.
doi: 10.1021/ja074841i |
| [23] |
Usta, H.; Risko, C.; Wang, Z.; Huang, H.; Deliomeroglu, M. K.; Zhukhovitskiy, A.; Facchetti, A.; Marks, T. J. J. Am. Chem. Soc. 2009, 131, 5586.
doi: 10.1021/ja809555c |
| [24] |
Fan, Z. P.; Li, X. Y.; Luo, X. E.; Fei, X.; Sun, B.; Chen, L. C.; Shi, Z. F.; Sun, C. L.; Shao, X. F.; Zhang, H. L. Adv. Funct. Mater. 2017, 27, 1702318.
doi: 10.1002/adfm.201702318 |
| [25] |
Sung, H.; Lin, H. Macromolecules 2004, 37, 7945.
doi: 10.1021/ma0489927 |
| [26] |
Hou, J. H.; Tan, Z. A.; Yan, Y.; He, Y. J.; Yang, C. H.; Li, Y. F. J. Am. Chem. Soc. 2006, 128, 4911.
doi: 10.1021/ja060141m |
| [27] |
Zhao, C. C.; Zhang, Y.; Ng, M. K. J. Org. Chem. 2007, 72, 6364.
doi: 10.1021/jo0704311 |
| [28] |
Rose, B. D.; Santa Maria, P. J., Fix, A. G.; Vonnegut, C. L.; Zakharov, L. N.; Parkin, S. R. Michael M. H. Beilstein J. Org. Chem. 2014, 10, 2122.
doi: 10.3762/bjoc.10.219 |
| [29] |
Wang, J.; Zeng, W. L.; Xu, H.; Li, B.; Cao, X. P.; Zhang, H. L. Chin. J. Chem. 2012, 30, 681.
doi: 10.1002/cjoc.201100141 |
| [30] |
Gao, X. K.; Wu, W. P.; Liu, Y. Q.; Jiao, S. B.; Qiu, W. F.; Yu, G.; Wang, L. P.; Zhu, D. B. J. Mater. Chem. 2007, 17, 736
doi: 10.1039/B613093C |
| [31] |
Romain, M.; Chevrier, M.; Bebiche, S.; Mohammed-Brahim, T.; Rault-Berthelot, J.; Jacques, E.; Poriel, C. J. Mater. Chem. C 2015, 3, 5742.
doi: 10.1039/C5TC00355E |
| [32] |
Kobayashi, N.; Sasaki, M.; Nomoto, K. Chem. Mater. 2009, 21, 552.
doi: 10.1021/cm802826m |
| [1] | Qifan Wang, Yuanquan Zhang, Li Xing, Yuanxiang Zhou, Chenyu Gong, Bangcan He, Nian Zhang, Yongjun Wu, Wei Xue. Design, Synthesis and Biological Activity of Myricetin Derivatives Containing 1,2,4-Triazolo[3,4-b]-1,3,4-thiadiazole [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1525-1536. |
| [2] | Chensheng Yu, Han Wang, Lijing Min, Liang Han, Jianjun Shi, Xinghai Liu. Synthesis, Cyrstal Structure and Fungicidal Activity of New Triazole Compounds Containing Trifluoromethylphenyl Moiety [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4498-4503. |
| [3] | He Mei, He Chaofan, Liu Ling, Ye Jiao, Hu Aixi, Chen Yun, Xu Lujie, Liu Ailin. Synthesis, Crystal Structure and Neuraminidase Inhibitory Activity of 1,2,4-Triazole-3-sulfide Derivatives [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2402-2410. |
| [4] | Cai Jinfang, Jiang Hua, Cui Zhihua, Chen Weiguo. Research Progress in Design, Synthesis and Application for Quinoidal Heterocyclic Compounds [J]. Chinese Journal of Organic Chemistry, 2020, 40(2): 351-363. |
| [5] | Jia Huijie, Han Limin, Zhu Ning, Gao Yuanyuan, Wang Yaqi, Suo Quanling. A Study on Recognition Property of Acetylferrocenyl Benzothiazole to Al3+, Cr3+ and Fe3+ [J]. Chin. J. Org. Chem., 2019, 39(6): 1753-1760. |
| [6] | Zhao Bangtun, Fu Huimin, Chen Xiaoji, Zhu Weimin. A New Method for Synthesizing Tetrathiafulvalene Vinylogues [J]. Chin. J. Org. Chem., 2018, 38(8): 2116-2121. |
| [7] | Shi Juan, Zhang Zunting. Synthesis and Fluorescent Property of Benzo [c]coumarin Carboxylic Derivatives [J]. Chin. J. Org. Chem., 2018, 38(6): 1462-1468. |
| [8] | Zhang Weijie, Xu Li, Song Jinsheng, Ma Zhiying, Wang Hua. Synthesis of Saddle-Shaped Cyclooctatetrathiophene-Triazine Derivatives and Their Aggregation Induced Emissions (AIE) Properties [J]. Chin. J. Org. Chem., 2018, 38(5): 1119-1125. |
| [9] | Mroz Robert, Vezzu Dileep A. K., Wallace Brian, Ravindranathan Deepak, Carroll Jeffrey, Pike Robert D., Huo Shouquan. A Comparative Study on Phosphorescent Cycloplatinated Complexes Based on Tridentate C^N^N-Coordinating Ligands and Phenylethynyl or Phenyl Ligand [J]. Chin. J. Org. Chem., 2018, 38(1): 171-182. |
| [10] | Jiang Haifang, Zhang Min, Zhang Li, Chen Yali, Zhu Ning, Song Liping, Deng Hongmei. Synthesis of 3,4-Diaryl-5-aryloxymethyl Isoxazole Derivatives [J]. Chin. J. Org. Chem., 2017, 37(9): 2399-2408. |
| [11] | Zhang Meimei, Wang Jie, Liu Jianquan, Wang Xiangshan. One-Pot and Three-Component Synthesis of Furopyridoquinoxaline Derivatives under Catalyst-Free Conditions [J]. Chin. J. Org. Chem., 2017, 37(6): 1565-1570. |
| [12] | Chen Sufang, Hong Yubiao, Liu Yuanzhong, Xue Mingqiang, Zheng Yu, Shen Qi. Synthesis and Properties of Sodium and Europium(Ⅲ) Cryptates Incorporating the 4,4'-Substituted-2,2'-bipyridine Units [J]. Chin. J. Org. Chem., 2017, 37(5): 1198-1204. |
| [13] | Zhang Wenlong, Chen Dongmei, Liu Xingli, Huang Chao, Zhu Bixue. Synthesis, Structure and Anion Recognition of Urea-Functionalized Schiff Base Macrocyclic Compound [J]. Chin. J. Org. Chem., 2017, 37(2): 474-479. |
| [14] | Ou Yaping, Zhang Jing, Yu Jiangxi, Zhu Xiaoming. Synthesis, Spectral Properties and Theoretical Studies of 1,4-Bis[2-(4-pyridyl)ethenyl]benzene Derivatives [J]. Chin. J. Org. Chem., 2017, 37(2): 394-402. |
| [15] | Ren Yaping, Liu Xu, Wang Rui, Zhou Yuanqing, Li Biao, Xu Yan, Song Maoping. Synthesis and Properties of 4-Ferrocenyl-benzoyl-thiadiazole Derivatives [J]. Chin. J. Org. Chem., 2017, 37(1): 110-115. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||