Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (1): 242-250.DOI: 10.6023/cjoc202307003 Previous Articles Next Articles
ARTICLES
徐利军a,b, 李宗军c, 韩福社a,b,*( ), 高翔a,b,*(
), 高翔a,b,*( )
)
收稿日期:2023-07-08
修回日期:2023-08-27
发布日期:2023-09-08
基金资助:
Lijun Xua,b, Zongjun Lic, Fushe Hana,b( ), Xiang Gaoa,b(
), Xiang Gaoa,b( )
)
Received:2023-07-08
Revised:2023-08-27
Published:2023-09-08
Contact:
*E-mail: Supported by:Share
Lijun Xu, Zongjun Li, Fushe Han, Xiang Gao. N,N-Dimethylformamide-Promoted Synthesis of Fullerene-Fused Oxazoline Derivatives[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 242-250.
| Entry | Amide (1a)/equiv. | Solvent | TBAOH/equiv. | I2/equiv. | Temp./℃ | Time/h | Yieldb/% of 2a |
|---|---|---|---|---|---|---|---|
| 1 | 80 | o-DCB | 3 | 2 | 30 | 2 | 0 |
| 2 | 80 | V(o-DCB)∶V(DMF)=1∶1 | 3 | 2 | 30 | 2 | Trace |
| 3 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 3 | 2 | 30 | 2 | 9 |
| 4 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 2 | 30 | 2 | 13 |
| 5 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 2 | 30 | 2 | Trace |
| 6 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 5 | 30 | 2 | 13 |
| 7 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 5 | 30 | 2 | 17 |
| 8 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 30 | 2 | 19 |
| 9 | 80 | V(o-DCB)∶V(CH3CN)=3∶1 | 10 | 10 | 30 | 2 | 0 |
| 10 | 80 | V(o-DCB)∶V(THF)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 11 | 80 | V(o-DCB)∶V(DMSO)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 12 | 80 | V(o-DCB)∶V(Toluene)=3∶1 | 10 | 10 | 30 | 2 | 3 |
| 13 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 2 | 25 |
| 14 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 90 | 2 | 0 |
| 15 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| 16 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 0.5 | 23 |
| 17 | 40 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 25 |
| 18 | 100 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| Entry | Amide (1a)/equiv. | Solvent | TBAOH/equiv. | I2/equiv. | Temp./℃ | Time/h | Yieldb/% of 2a |
|---|---|---|---|---|---|---|---|
| 1 | 80 | o-DCB | 3 | 2 | 30 | 2 | 0 |
| 2 | 80 | V(o-DCB)∶V(DMF)=1∶1 | 3 | 2 | 30 | 2 | Trace |
| 3 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 3 | 2 | 30 | 2 | 9 |
| 4 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 2 | 30 | 2 | 13 |
| 5 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 2 | 30 | 2 | Trace |
| 6 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 5 | 5 | 30 | 2 | 13 |
| 7 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 5 | 30 | 2 | 17 |
| 8 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 30 | 2 | 19 |
| 9 | 80 | V(o-DCB)∶V(CH3CN)=3∶1 | 10 | 10 | 30 | 2 | 0 |
| 10 | 80 | V(o-DCB)∶V(THF)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 11 | 80 | V(o-DCB)∶V(DMSO)=3∶1 | 10 | 10 | 30 | 2 | 13 |
| 12 | 80 | V(o-DCB)∶V(Toluene)=3∶1 | 10 | 10 | 30 | 2 | 3 |
| 13 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 2 | 25 |
| 14 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 90 | 2 | 0 |
| 15 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
| 16 | 80 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 0.5 | 23 |
| 17 | 40 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 25 |
| 18 | 100 | V(o-DCB)∶V(DMF)=3∶1 | 10 | 10 | 70 | 1 | 31 |
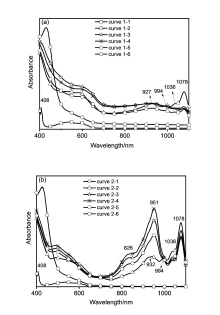



| Compound | E1red a/V | εLUMOb/eV |
|---|---|---|
| 2a | -0.99 | -3.81 |
| 2c | -1.07 | -3.73 |
| 2f | -1.01 | -3.79 |
| 2g | -1.00 | -3.80 |
| 2i | -1.01 | -3.79 |
| 2j | -0.99 | -3.81 |
| C60 | -0.97 | -3.83 |
| Compound | E1red a/V | εLUMOb/eV |
|---|---|---|
| 2a | -0.99 | -3.81 |
| 2c | -1.07 | -3.73 |
| 2f | -1.01 | -3.79 |
| 2g | -1.00 | -3.80 |
| 2i | -1.01 | -3.79 |
| 2j | -0.99 | -3.81 |
| C60 | -0.97 | -3.83 |
| [1] |
Zarrabi, N.; Seetharaman, S.; Chaudhuri, S.; Holzer, N.; Batista, V. S.; Van Der Est, A.; D’Souza, F.; Poddutoori, P. K. J. Am. Chem. Soc. 2020, 142, 10008.
doi: 10.1021/jacs.0c01574 |
| [2] |
Sun, C.; Yang, P.-P.; Nan, Z.; Tian, C.-B; Cai, Y.-T.; Chen, J.-F.; Qi, F.-F.; Tian, H.-R.; Xie, L.-Q.; Meng, L.-Y.; Wei, Z.-H. Adv. Mater. 2023, 35, 2205603.
doi: 10.1002/adma.v35.9 |
| [3] |
Li, S.-H.; Xing, Z.; Wu, B.-S.; Chen, Z.-C.; Yao, Y.-R.; Tian, H.-R.; Li, M.-F.; Yun, D.-Q.; Deng, L.-L.; Xie, S.-Y.; Huang, R.-B.; Zheng, L.-S. ACS Appl. Mater. Interfaces 2020, 12, 20733.
doi: 10.1021/acsami.0c02119 |
| [4] |
Fan, J.-Q.; Fang, G.; Zeng, F.; Wang, X.-D.; Wu, S.-Z. Small 2013, 9, 613.
doi: 10.1002/smll.v9.4 |
| [5] |
Li, Y.-B.; Biswas, R.; Kopcha, W. P.; Dubroca, T.; Abella, L.; Sun, Y.; Crichton, R. A.; Rathnam, C.; Yang, L.-T.; Yeh, Y.-W.; Kundu, K.; Rodríguez-Fortea, A.; Poblet, J. M.; Lee, K.-B.; Hill, S.; Zhang, J.-Y. Angew. Chem., Int. Ed. 2023, 62, e202211704.
|
| [6] |
Ramos-Soriano, J.; Reina, J. J.; Illescas, B. M.; de la Cruz, N.; Rodríguez-Pérez, L.; Lasala, F.; Rojo, J.; Delgado, R.; Martín, N. J. Am. Chem. Soc. 2019, 141, 15403.
doi: 10.1021/jacs.9b08003 |
| [7] |
Zhu, S.-E.; Dou, L.-F.; Zhang, J.-H.; Wu, Y.; Yang, W.; Lu, H.-D.; Wei, C.-X.; Deng, C.-H.; Dong, Q. Chin. J. Org. Chem. 2021, 41, 2082 (in Chinese).
doi: 10.1002/cjoc.v41.17 |
|
(朱三娥, 豆礼锋, 张建辉, 吴缨, 杨伟, 鲁红典, 卫春祥, 邓崇海, 董强, 有机化学, 2021, 41, 2082.)
|
|
| [8] |
Niu, C.; Liu, Z.; Chen, M.-Q.; Yang, S.-F.; Wang, G.-W. Org. Lett. 2022, 24, 3493.
doi: 10.1021/acs.orglett.2c01097 |
| [9] |
Avila, L. B.; Serrano Arambulo, P. C.; Dantas, A.; Cuevas-Arizaca, E. E.; Kumar, D.; Müller, C. K. Nanomaterials 2022, 12, 2881.
doi: 10.3390/nano12162881 |
| [10] |
Yan, X.-X.; Niu, C.; Yin, Z.-C.; Lu, W.-Q.; Wang, G.-W. Sci. Bull. 2022, 67, 2406.
doi: 10.1016/j.scib.2022.11.023 |
| [11] |
Niu, C.; Wang, G.-W. Chin. J. Org. Chem. 2020, 40, 3633 (in Chinese).
doi: 10.6023/cjoc202006081 |
|
(牛闯, 王官武, 有机化学, 2020, 40, 3633.)
|
|
| [12] |
Hashiguchi, M.; Obata, N.; Maruyama, M.; Yeo, K. S.; Ueno, T.; Ikebe, T.; Takahashi, I.; Matsuo, Y. Org. Lett. 2012, 14, 3276.
doi: 10.1021/ol301186u |
| [13] |
Thiesbrummel, J.; Peña-Camargo, F.; Brinkmann, K. O.; Gutierrez- Partida, E.; Yang, F.-J.; Warby, J.; Albrecht, S.; Neher, D.; Riedl, T.; Snaith, H. J.; Stolterfoht, M.; Lang, F. Adv. Energy Mater. 2023, 13, 2202674.
doi: 10.1002/aenm.v13.3 |
| [14] |
Guo, Y.; Zhu, H.-X.; Liu, G.-L.; Yan, H.-M.; Zhu, B.-J.; Li, S.; Sun, Y.-J.; Li, G.-H. Chin. J. Org. Chem. 2016, 36, 172 (in Chinese).
|
|
(郭颖, 朱华新, 刘桂林, 严慧敏, 朱冰洁, 李帅, 孙亚军, 李果华, 有机化学, 2016, 36, 172.)
|
|
| [15] |
(a) Yang, W.-W.; Li, Z.-J.; Li, F.-F.; Gao, X. J. Org. Chem. 2011, 76, 1384.
doi: 10.1021/jo1023798 |
|
(b) Li, Z.-J.; Li, S.-H.; Sun, T.; Hou, H.-L.; Gao, X. J. Org. Chem. 2015, 80, 3566.
doi: 10.1021/acs.joc.5b00253 |
|
|
(c) Liu, Q.-S.; Qiu, W.-J.; Lu, W.-Q.; Wang, G.-W. Org. Biomol. Chem. 2022, 20, 3535.
doi: 10.1039/D2OB00239F |
|
| [16] |
(a) Banks, M. R.; Cadogan, J. I. G.; Gosney, I.; Hodgson, P. K. G.; Langridge-Smith, P. R. R.; Rankine, D. W. H. J. Chem. Soc., Chem. Commun. 1994, 25, 1365.
|
|
(b) Banks, M. R.; Cadogan, J. I. G.; Gosney, I.; Hodgson, P. K. G.; Langridge-Smith, P. R. R.; Millar, J. R. A.; Taylor, A. T. Tetrahedron Lett. 1994, 35, 9067.
doi: 10.1016/0040-4039(94)88429-3 |
|
| [17] |
Averdung, J.; Mattay, J.; Jacobi, D.; Abraham, W. Tetrahedron 1995, 51, 2543.
doi: 10.1016/0040-4020(95)00013-X |
| [18] |
Yang, H.-T.; Xing, M.-L.; Zhu, Y.-F.; Sun, X.-Q.; Cheng, J.; Miao, C.-B.; Li, F.-B. J. Org. Chem. 2014, 79, 1487.
doi: 10.1021/jo4025573 |
| [19] |
(a) Zheng, M.; Li, F.-F.; Ni, L.; Yang, W.-W.; Gao, X. J. Org. Chem. 2008, 73, 3159.
doi: 10.1021/jo702678c |
|
(b) Hou, H.-L. Gao, X. J. Org. Chem. 2012, 77, 2553.
doi: 10.1021/jo202616j |
|
| [20] |
Chang, W.-W.; Li, Z.-J.; Yang, W.-W.; Gao, X. Org. Lett. 2012, 14, 2386.
doi: 10.1021/ol300805p |
| [21] |
(a) Li, F.-B.; Liu, T.-X.; Wang, G.-W. J. Org. Chem. 2008, 73, 6417.
doi: 10.1021/jo8007868 |
|
(b) Yang, H.-T.; Liang, X.-C.; Wang, Y.-H.; Yang, Y.; Sun, X.-Q.; Miao, C.-B. Org. Lett. 2013, 15, 4650.
doi: 10.1021/ol401909z |
|
|
(c) Zhang, X.-F.; Li, F.-B.; Shi, J.-L.; Wu, J.; Liu, L. New J. Chem. 2016, 40, 1626.
doi: 10.1039/C5NJ02503F |
|
|
(d) Liu, T.-X.; Liu, Y.-Q.; Chao, D.; Zhang, P.-L.; Liu, Q.-F.; Shi, L.; Zhang, Z.-G.; Zhang, G.-S. J. Org. Chem. 2014, 79, 11084.
doi: 10.1021/jo5020883 |
|
|
(e) Liu, Q.-S.; Qiu, W.-J.; Lu, W.-Q.; Wang, G.-W. Org. Biomol. Chem. 2022, 20, 3535.
doi: 10.1039/D2OB00239F |
|
| [22] |
Takeda, Y.; Enokijima, S.; Nagamachi, T.; Nakayama, K.; Minakata, S. Asian J. Org. Chem. 2013, 2, 91.
doi: 10.1002/ajoc.v2.1 |
| [23] |
Yang, H.-T.; Ren, W.-L.; Dong, C.-P.; Yang, Y.; Sun, X.-Q.; Miao, C.-B. Tetrahedron Lett. 2013, 54, 6799.
doi: 10.1016/j.tetlet.2013.09.002 |
| [24] |
Rosén, A.; Wästberg, B. J. Chem. Phys. 1989, 90, 2525.
doi: 10.1063/1.455947 |
| [25] |
Xie, Q.-S.; Perez-Cordero, E.; Echegoyen, L. J. Am. Chem. Soc. 1992, 114, 3978.
doi: 10.1021/ja00036a056 |
| [26] |
(a) Fagan, P. J.; Krusic, P. J.; Evans, D. H.; Lerke, S. A.; Johnston, E. J. Am. Chem. Soc. 1992, 114, 9697.
doi: 10.1021/ja00050a081 |
|
(b) Schick, G.; Kampe, K. D.; Hirsch, A. J. Chem. Soc., Chem. Commun. 1995, 19, 2023.
|
|
|
(c) Wang, G.-W.; Shu, L.-H.; Wu, S.-H.; Wu, H.-M.; Lao, X.-F. J. Chem. Soc., Chem. Commun. 1995, 10, 1071.
|
|
| [27] |
Matsuo, Y.; Iwashita, A.; Abe, Y.; Li, C.-Z.; Matsuo, K.; Hashiguchi, M.; Nakamura, E. J. Am. Chem. Soc. 2008, 130, 15429.
doi: 10.1021/ja8041299 |
| [28] |
Isobe, H.; Tanaka, T.; Nakanishi, W.; Lemiègre, L.; Nakamura, E. J. Org. Chem. 2005, 70, 4826.
doi: 10.1021/jo050432y |
| [29] |
Chang, W.-W.; He, F.-G.; Garcıa-Penas, A.; Shekh, M. I.; Li, Z.-J. RSC Adv. 2022, 12, 14018.
doi: 10.1039/D2RA01300B |
| [30] |
Naim, A.; Shevlin, P. B. Tetrahedron Lett. 1992, 33, 7097.
doi: 10.1016/S0040-4039(00)60845-6 |
| [31] |
Hare, J. P.; Kroto, H. W.; Taylor, R. Chem. Phys. Lett. 1991, 177, 394.
doi: 10.1016/0009-2614(91)85072-5 |
| [32] |
Khaled, M. M.; Carlin, R. T.; Trulove, P. C.; Eaton, G. R.; Eaton, S. S. J. Am. Chem. Soc. 1994, 116, 3465.
doi: 10.1021/ja00087a037 |
| [33] |
Rapta, P.; Bartl, A.; Gromov, A.; Stasko, A.; Dunsch, L. ChemPhyChem 2002, 3, 351.
|
| [34] |
Subramanian, R.; Kadish, K. M.; Vijayashree, M. N.; Gao, X.; Jones, M. T.; Miller, M. D.; Krause, K. L.; Suenobu, T.; Fukuzumi, S. J. Phys. Chem. 1996, 100, 16327.
doi: 10.1021/jp961412q |
| [35] |
Xu, L.-J.; Yang, W.-W.; Han, F.-S.; Gao, X. Org. Biomol. Chem. 2023, 21, 2331.
doi: 10.1039/D3OB00039G |
| [36] |
(a) Li, F.-F.; Gao, X.; Zheng, M. J. Org. Chem. 2009, 74, 82.
doi: 10.1021/jo801769q |
|
(b) Yang, W.-W.; Li, Z.-J.; Li, S.-H.; Gao, X. J. Phys. Chem. A 2015, 119, 9534.
doi: 10.1021/acs.jpca.5b07932 |
|
| [37] |
(a) Liu, Z.-J.; Larock, R. C. J. Am. Chem. Soc. 2005, 127, 13112.
doi: 10.1021/ja054079p |
|
(b) Sukata, K. Bull. Chem. Soc. Jpn. 1985, 58, 838.
doi: 10.1246/bcsj.58.838 |
|
| [38] |
Zhao, H.; Zhu, X.-Y.; Hu, X.-X.; Liu, Y.-G.; Tang, C.-L.; Feng, B.-N. Chin. J. Org. Chem. 2019, 39, 434 (in Chinese).
doi: 10.6023/cjoc201807010 |
|
(赵辉, 朱孝云, 胡小霞, 刘延革, 唐春雷, 冯柏年, 有机化学, 2019, 39, 434.)
|
|
| [39] |
Hou, H.-L.; Li, Z.-J.; Li, S.-H.; Chen, S.; Gao, X. Org. Lett. 2013, 15, 4646.
doi: 10.1021/ol401834j |
| [40] |
Zhuo, L.-G.; Liao, W.; Yu, Z.-X. Asian J. Org. Chem. 2012, 1, 336.
doi: 10.1002/ajoc.v1.4 |
| [41] |
(a) Li, D.; Li, Z.-J.; He, F.-G.; Geng, C.; Gao, X. J. Org. Chem. 2019, 84, 14679.
doi: 10.1021/acs.joc.9b02272 |
|
(b) Yang, S.-T.; Zhou, X.-Y.; Hu, Y.-J.; Abella, L.; Yao, Y.-R.; Peng, P.; Zhang, Q.-Y.; Rodríguez-Fortea, A.; Poblet, J. M.; Li, F.-F. J. Org. Chem. 2023, 88, 4234.
doi: 10.1021/acs.joc.2c02779 |
|
| [42] |
Li, X.-J.; Li, Y.-F. Acta Polym. Sin. 2022, 53, 995 (in Chinese).
|
|
(李骁骏, 李永舫, 高分子学报, 2022, 53, 995.)
|
| [1] | Zhaoming Hu, Jihong Wu, Jingjing Wu, Fanhong Wu. Research Progress on Direct Trifluoromethylselenylation [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 36-56. |
| [2] | Guangyu Zhang, Jiaxi Xu. [3,3] Sigmatropic Shifts and Applications of Hydroxylamine Derivatives [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3002-3014. |
| [3] | Wenhao Yuan, Jiaxi Xu. Ring Expansions of Oxetanes [J]. Chinese Journal of Organic Chemistry, 2021, 41(3): 947-958. |
| [4] | Tao Xuefen, Sheng Rong, Bao Kun, Wang Yuxin, Jin Yinxiu. Progress of Difluoromethyl Heteroaryl Sulfones as Difluoroalkylation Reagents [J]. Chinese Journal of Organic Chemistry, 2019, 39(10): 2726-2734. |
| [5] | Deng Jun, Fu Xiangkai, Wang Gang, Huang Jing, Wu Liu, Zou Xiaochuan. Progress in Synthesis and Application of 2,5-Dithienylpyrrole Derivatives [J]. Chin. J. Org. Chem., 2012, 32(06): 1051-1059. |
| [6] | LV Xin-Ming , QIAN Ying. Synthesis and Photophysical Properties of a Novel Hyperbranched Fluorescence Polymer [J]. Chin. J. Org. Chem., 2011, 31(01): 82-86. |
| [7] | LI Xue-Qiang, WEI Chuan-Wan, LIU Xiao-Fang, LIU You-Nian. Synthesis of Ferrocenoyl-peptide and Its Inhibition for β-Amyloid Peptide [J]. Chin. J. Org. Chem., 2010, 30(10): 1492-1496. |
| [8] | Li, Hongqi*,a; Song, Yanxib ; Li, Xiumeic. Advances in Synthesis and Research of Tetrathiafulvalenes Containing Halogen Substituent [J]. Chin. J. Org. Chem., 2009, 29(06): 858-866. |
| [9] | Xiao, Ling ; Zeng, Heping*. Synthesis, Electrochemical and Two-Photon Absorption Properties of N-Methyl-2-{N-ethyl-6-[2-(8-methoxylquinol-2-yl)vinyl]carbazol-3-yl}- fulleropyrrolidine [J]. Chin. J. Org. Chem., 2009, 29(05): 742-747. |
| [10] | LI Hong-Qi*,a,SONG Yan-Xib,PENG Jia-Jianc,QIU Hua-Yuc. Advances in Synthesis and Research of Macrocyclic Compounds Containing Tetrathiafulvalene [J]. Chin. J. Org. Chem., 2007, 27(10): 1220-1227. |
| [11] |
YANG Xin-Lin*,a,QIAO Xin-Gea,ZHU Zhang-Guanga,CHENG Fu-Yongb ZHU He-Sunb,FAN Lou-Zhenc. Synthesis and Electrochemical Properties of Mono- methano[60]fullerene Organophosphonates [J]. Chin. J. Org. Chem., 2006, 26(11): 1557-1561. |
| [12] | Cui Dajun;Peng Lijun;Zeng Xianshun;Xu Fengbo;Zhang Zhengzhi. New Substitution Process for Preparation of trans-Fe(CO)_3(PR_3)_2 [J]. Chin. J. Org. Chem., 2003, 23(9): 973-976. |
| [13] | Ruan Jiwu;Huang Zhongjing;Fu Liwu;Ma Lin;Gu Lianquan. Study on the Nucleophilic Substitutions of 4,4'-Difluorobenzil with Amines [J]. Chin. J. Org. Chem., 2003, 23(8): 861-864. |
| [14] | Xuan Guangrong;He Ningde. Reaction of 7,7-Dichlorobicyclo[4,1,0]heptane wth Active Methylene Compounds [J]. Chin. J. Org. Chem., 2003, 23(7): 734-736. |
| [15] | Wang Jianwu;Jia Jiong;Hou Dianjie;Li Hongmei;Yin Jun. A novel tandem reaction to synthesize imidazo [l ,5-α] pyridine derivatives [J]. Chin. J. Org. Chem., 2003, 23(2): 173-175. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||