Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (1): 232-241.DOI: 10.6023/cjoc202305025 Previous Articles Next Articles
ARTICLES
王博珍a,b, 张婕b, 粘春惠b, 金茗茗b, 孔苗苗c, 李物兰c, 何文斐b,*( ), 吴建章a,b,*(
), 吴建章a,b,*( )
)
收稿日期:2023-05-18
修回日期:2023-08-16
发布日期:2023-09-15
作者简介:基金资助:
Bozhen Wanga,b, Jie Zhangb, Chunhui Nianb, Mingming Jinb, Miaomiao Kongc, Wulan Lic, Wenfei Heb( ), Jianzhang Wua,b(
), Jianzhang Wua,b( )
)
Received:2023-05-18
Revised:2023-08-16
Published:2023-09-15
Contact:
*E-mail: About author:Supported by:Share
Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides[J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241.
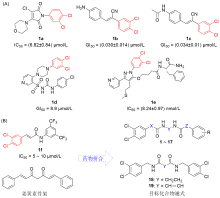

| Compd. | X | Z | Y | R | Growth inhibition/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AGS | BGC-823 | |||||||||
| 1 | CH=CH | CH=CH | — | 2-OCH3 | 49.43±2.43 | NA | ||||
| 2 | CH=CH | CH=CH | — | 2-Br | 42.28±0.12 | NA | ||||
| 3 | CH=CH | CH=CH | — | 2-Cl | 32.52±1.39 | NA | ||||
| 4 | CH=CH | CH=CH | — | 2-CF3 | 46.59±2.28 | 17.83±0.40 | ||||
| 5 | CH=CH | CH=CH | — | 2-NO2 | 33.56±0.19 | NA | ||||
| 6 | CH=CH | CH=CH | — | 2,3-(OCH3)2 | 38.96±1.58 | 8.98±0.52 | ||||
| 7 | CH=CH | CH=CH | — | 2,4-(OCH3)2 | 29.87±1.86 | 6.90±0.77 | ||||
| 8 | CH=CH | CH=CH | — | 2,4-Me2 | 34.41±0.69 | NA | ||||
| 9 | CH=CH | CH=CH | — | 3,4-Me2 | 44.41±2.56 | NA | ||||
| 10 | CH=CH | CH=CH | — | 4-N(Me)2 | 48.77±2.12 | NA | ||||
| 11 | CH=CH | CH=CH | — | | 48.78±2.36 | NA | ||||
| 12 | CH=CH | CH=CH | — | | 43.46±1.23 | NA | ||||
| 13 | CH2 | CH2 | | 3,4-Cl2 | NA | 6.14±1.77 | ||||
| 14 | CH=CH | CH=CH | | 3,4-Cl2 | 41.10±1.09 | 2.78±1.76 | ||||
| 15 | — | — | (CH2)4 | 3,4-Cl2 | 44.65±1.63 | 26.32±1.89 | ||||
| 16 | CH=CH | (CH2)2 | — | 3,4-Cl2 | 76.75±2.10 | 3.87±1.94 | ||||
| 17 | CH=CH | (CH2)2 | — | 4-CF3 | 83.95±0.35 | 45.66±0.81 | ||||
| 18 | | 4.95±0.64 | 6.67±2.51 | |||||||
| 19 | | 37.45±1.48 | 20.05±1.91 | |||||||
| 1f | | 64.77±0.01 | 36.16±0.04 | |||||||
| Curcuminb | | 70.43±8.89 | 38.52±6.64 | |||||||
| Compd. | X | Z | Y | R | Growth inhibition/% | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AGS | BGC-823 | |||||||||
| 1 | CH=CH | CH=CH | — | 2-OCH3 | 49.43±2.43 | NA | ||||
| 2 | CH=CH | CH=CH | — | 2-Br | 42.28±0.12 | NA | ||||
| 3 | CH=CH | CH=CH | — | 2-Cl | 32.52±1.39 | NA | ||||
| 4 | CH=CH | CH=CH | — | 2-CF3 | 46.59±2.28 | 17.83±0.40 | ||||
| 5 | CH=CH | CH=CH | — | 2-NO2 | 33.56±0.19 | NA | ||||
| 6 | CH=CH | CH=CH | — | 2,3-(OCH3)2 | 38.96±1.58 | 8.98±0.52 | ||||
| 7 | CH=CH | CH=CH | — | 2,4-(OCH3)2 | 29.87±1.86 | 6.90±0.77 | ||||
| 8 | CH=CH | CH=CH | — | 2,4-Me2 | 34.41±0.69 | NA | ||||
| 9 | CH=CH | CH=CH | — | 3,4-Me2 | 44.41±2.56 | NA | ||||
| 10 | CH=CH | CH=CH | — | 4-N(Me)2 | 48.77±2.12 | NA | ||||
| 11 | CH=CH | CH=CH | — | | 48.78±2.36 | NA | ||||
| 12 | CH=CH | CH=CH | — | | 43.46±1.23 | NA | ||||
| 13 | CH2 | CH2 | | 3,4-Cl2 | NA | 6.14±1.77 | ||||
| 14 | CH=CH | CH=CH | | 3,4-Cl2 | 41.10±1.09 | 2.78±1.76 | ||||
| 15 | — | — | (CH2)4 | 3,4-Cl2 | 44.65±1.63 | 26.32±1.89 | ||||
| 16 | CH=CH | (CH2)2 | — | 3,4-Cl2 | 76.75±2.10 | 3.87±1.94 | ||||
| 17 | CH=CH | (CH2)2 | — | 4-CF3 | 83.95±0.35 | 45.66±0.81 | ||||
| 18 | | 4.95±0.64 | 6.67±2.51 | |||||||
| 19 | | 37.45±1.48 | 20.05±1.91 | |||||||
| 1f | | 64.77±0.01 | 36.16±0.04 | |||||||
| Curcuminb | | 70.43±8.89 | 38.52±6.64 | |||||||



| [1] |
Sung, H.; Ferlay, J.; Siegel, R. L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Ca-Cancer J. Clin. 2021, 71, 209.
doi: 10.3322/caac.v71.3 |
| [2] |
Tsai, C. H.; Lin, Y. H.; Li, Y. S.; Ho, T. L.; Hoai Thuong, L. H.; Liu, Y. H. Int. J. Mol. Sci. 2021, 22, 9257.
doi: 10.3390/ijms22179257 |
| [3] |
Margiotta, E.; van der Lubbe, S. C. C.; de Azevedo Santos, L.; Paragi, G.; Moro, S.; Bickelhaupt, F. M.; Fonseca Guerra, C. J. Chem. Inf. Model. 2020, 60, 1317.
doi: 10.1021/acs.jcim.9b00946 |
| [4] |
Wilcken, R.; Zimmermann, M. O.; Lange, A.; Joerger, A. C.; Boeckler, F. M. J. Med. Chem. 2013, 56, 1363.
doi: 10.1021/jm3012068 |
| [5] |
Fang, W. Y.; Ravindar, L.; Rakesh, K. P.; Manukumar, H. M.; Shantharam, C. S.; Alharbi, N. S.; Qin, H. L. Eur. J. Med. Chem. 2019, 173, 117.
doi: 10.1016/j.ejmech.2019.03.063 |
| [6] |
Koksal, M.; Yarim, M.; Erdal, A.; Bozkurt, A. Drug Res. (Stuttgart, Ger.) 2014, 64, 66.
doi: 10.1055/s-00023610 |
| [7] |
Koksal, M.; Bilge, S. S.; Bozkurt, A.; Sahin, Z. S.; Isik, S.; Erol, D. D. Arzneimittelforschung 2008, 58, 510.
doi: 10.1055/s-0031-1296550 |
| [8] |
Scozzafava, A.; Mastrolorenzo, A.; Supuran, C. T. J. Enzyme Inhib. 2001, 16, 425.
|
| [9] |
Onda, K.; Shiraki, R.; Yonetoku, Y.; Momose, K.; Katayama, N.; Orita, M.; Yamaguchi, T.; Ohta, M.; Tsukamoto, S. Bioorg. Med. Chem. 2008, 16, 8627.
doi: 10.1016/j.bmc.2008.08.010 |
| [10] |
Budke, B.; Kalin, J. H.; Pawlowski, M.; Zelivianskaia, A. S.; Wu, M.; Kozikowski, A. P.; Connell, P. P. J. Med. Chem. 2013, 56, 254.
doi: 10.1021/jm301565b |
| [11] |
Baker, J. R.; Gilbert, J.; Paula, S.; Zhu, X.; Sakoff, J. A.; Mc-Clus- key, A. ChemMedChem 2018, 13, 1447.
doi: 10.1002/cmdc.v13.14 |
| [12] |
Szafrański, K.; Sławiński, J. Molecules 2015, 20, 12029.
doi: 10.3390/molecules200712029 |
| [13] |
Peterson, Y. K.; Kelly, P.; Weinbaum, C. A., Casey, P. J. J. Biol. Chem. 2006, 281, 12445.
doi: 10.1074/jbc.M600168200 |
| [14] |
Kumari, S.; Carmona, A. V.; Tiwari, A. K.; Trippier, P. C. J. Med. Chem. 2020, 63, 12290.
doi: 10.1021/acs.jmedchem.0c00530 |
| [15] |
Pattabiraman, V. R.; Bode, J. W. Nature 2011, 480, 471.
doi: 10.1038/nature10702 |
| [16] |
Tang, J.-J. M.S. Thesis, Hunan University, Changsha, 2017 (in Chinese).
|
|
(唐晶晶, 硕士论文, 湖南大学, 长沙, 2017.)
|
|
| [17] |
Strharsky, T.; Pindjakova, D.; Kos, J.; Vrablova, L.; Michnova, H.; Hosek, J.; Strakova, N.; Lelakova, V.; Leva, L.; Kavanova, L.; Oravec, M.; Cizek, A.; Jampilek, J. Int. J. Mol. Sci. 2022, 23, 3159.
doi: 10.3390/ijms23063159 |
| [18] |
Yu, P.; Hu, J.; Zhou, T. Y.; Wang, P.; Xu, Y. H. J. Chem. Res. 2011, 35, 703.
doi: 10.3184/174751911X13230201951890 |
| [19] |
Esterhuysen, C.; Heßelmann, A.; Clark, T. ChemPhysChem 2017, 18, 772.
doi: 10.1002/cphc.v18.7 |
| [20] |
Abula, A.; Xu, Z.; Zhu, Z.; Peng, C.; Chen, Z.; Zhu, W.; Aisa, H. A. J. Chem. Inf. Model. 2020, 60, 6242.
doi: 10.1021/acs.jcim.0c00898 |
| [21] |
He, X.-H.; Li, H.-P.; Zhao, Q.; Peng, C.; Huang, W. Chin. Arch. Tradit. Chin. Med. 2022, 40, 175 (in Chinese).
|
|
(何享鸿, 李和平, 赵倩, 彭成, 黄维, 中华中医药学刊, 2022, 40, 175.)
|
|
| [22] |
Rammohan, M.; Harris, E.; Bhansali, R. S.; Zhao, E.; Li, L. S.; Crispino, J. D. Oncogene 2022, 41, 2003.
doi: 10.1038/s41388-022-02245-6 |
| [23] |
Chen, Z. Y.; Li, J.; Zhu, S. D.; Li, Z. D.; Yu, J. L.; Wu, J.; Zhang, C.; Zeng, L. H. Exp. Ther. Med. 2022, 23, 209.
doi: 10.3892/etm |
| [1] | Penghui Li, Qingyang Xie, Fuxian Wan, Yuanhong Zhang, Lin Jiang. Synthesis and Fungicidal Activity of Novel Substituted Pyrimidine-5-carboxamides Bearing Cyclopropyl Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 650-656. |
| [2] | Fakai Zou, Nengzhong Wang, Hui Yao, Hui Wang, Mingguo Liu, Nianyu Huang. Regio- and Stereo-selective Synthesis of 1β-/3R-Aryl Thiosugar [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 593-604. |
| [3] | Luyao Li, Zhongwen He, Zhenguo Zhang, Zhenhua Jia, Teck-Peng Loh. Application of Triaryl Carbenium in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 421-437. |
| [4] | Qinggang Mei, Qinghan Li. Recent Progress of Visible Light-Induced the Synthesis of C(3) (Hetero)arylthio Indole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 398-408. |
| [5] | Shihang Yu, Jiawei Liu, Biyu An, Qinghua Bian, Min Wang, Jiangchun Zhong. Asymmetric Synthesis of the Contact Sex Pheromone of Neoclytus acuminatus acuminatus (Fabricius) [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 301-308. |
| [6] | Weiqing Yang, Yanbing Ge, Yuanyuan Chen, Ping Liu, Haiyan Fu, Menglin Ma. Design and Synthesis of Fluorescent 1,8-Napthalimide Derivatives and Their Identification of Cysteine [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 180-194. |
| [7] | Qianfan Zhao, Yongzheng Chen, Shiming Zhang. Application and Mechanism Study of Carbon-Based Metal-Free Catalysts in Organic Synthesis [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 137-147. |
| [8] | Shan Chen, Zhilin Chen, Qiong Hu, Yanshuang Meng, Yue Huang, Pingfang Tao, Liru Lu, Guobao Huang. Recognition of Bis-thiourea Tweezers to Neutral Molecules in Non-Polar Solvent [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 277-281. |
| [9] | Huakun Wang, Xiaolong Ren, Yining Xuan. Study of the Halide Salt Catalyzed [3+2] Cycloaddition of α,β-Epoxy Carboxylate with Isocyanate [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 251-258. |
| [10] | Yukun Jin, Baoyi Ren, Fushun Liang. Visible Light-Mediated Selective C—F Bond Cleavage of Trifluoromethyl Groups and Its Application in Synthesizing gem-Difluoro-Containing Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 85-110. |
| [11] | Cuiyun Ma, Hailan Luo, Fuhua Zhang, Dan Guo, Shuxing Chen, Fei Wang. Green Biosynthesis, Photophysical Properties and Application of 3-Pyrrolyl BODIPY [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 216-223. |
| [12] | Ruixia Cao, Yuping Jia. Synthesis and Biological Activity of Novel Pyrrolo[2,3-d]pyrimidine Derivatives Containing Coumarin [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3304-3311. |
| [13] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [14] | Ran Zhou, Chunmei Yuan, Tao Zhang, Piao Mao, Yi Liu, Kaini Meng, Hui Xin, Wei Xue. Design, Synthesis and Bioactivity of Chalcone Derivative Containing Quinazolinone [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3196-3209. |
| [15] | Yang Li, Jinding Yuan, Di Zhao. Deep Eutectic Solvent of 1,3-Dimethylurea/L-(+)-Tartaric Acid for the Green Synthesis of (E)-2-Styrylquinoline-3-carboxylic Acid Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3268-3276. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||