Chinese Journal of Organic Chemistry ›› 2024, Vol. 44 ›› Issue (6): 1949-1956.DOI: 10.6023/cjoc202311023 Previous Articles Next Articles
ARTICLES
梁国超a, 董婷婷a, 纪海莹a, 王春艳a, 宋亚丽b, 张伟a,*( )
)
收稿日期:2023-11-23
修回日期:2024-01-18
发布日期:2024-03-05
基金资助:
Guochao Lianga, Tingting Donga, Haiying Jia, Chunyan Wanga, Yali Songb, Wei Zhanga,*( )
)
Received:2023-11-23
Revised:2024-01-18
Published:2024-03-05
Contact:
* E-mail: Supported by:Share
Guochao Liang, Tingting Dong, Haiying Ji, Chunyan Wang, Yali Song, Wei Zhang. Synthesis and Antitumor Activity of Novel 3,3'-((4-Chloro-2H-thiochromen-3-yl)methylene)bis(1H-indole)-Like Topoisomerase II Inhibitors[J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1949-1956.
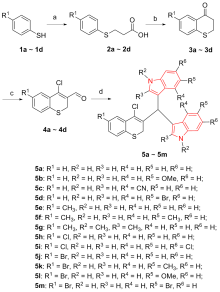

| Compd. | IC50/(μmol•L–1) | |||||
|---|---|---|---|---|---|---|
| HeLa | H446 | U-937 | MCF-7 | SK-OV-3 | HT-29 | |
| 5a | 16.5±1.2 | 17.6±3.2 | 12.7±1.1 | 17.7±2.8 | 18.6±2.1 | 19.8±1.9 |
| 5b | 14.5±2.2 | 8.6±1.4 | 10.3±1.9 | 14.2±1.7 | 16.8±1.9 | 14.1±3.4 |
| 5c | 22.4±2.7 | 18.8±2.0 | 11.9±2.4 | 10.5±2.2 | 15.4±1.6 | 12.2±2.3 |
| 5d | 38.4±3.3 | 65.6±3.6 | 72.7±4.1 | 47.5±2.5 | 66.6±2.4 | 72.6±3.9 |
| 5e | 20.5±1.5 | 28.9±1.4 | 21.4±2.1 | 65.1±4.2 | 41.5±2.7 | 25.3±3.2 |
| 5f | 8.7±1.2 | 8.8±2.1 | 14.3±3.1 | 14.9±1.7 | 13.3±2.7 | 21.1±1.6 |
| 5g | 10.6±2.3 | 12.8±1.4 | 10.5±1.7 | 7.4±1.8 | 14.7±3.6 | 12.7±2.3 |
| 5h | 66.7±2.6 | 32.5±1.7 | 45.7±4.5 | 34.6±2.2 | 64.3±3.9 | 22.6±2.4 |
| 5i | 45.2±3.6 | 37.8±3.1 | 35.2±2.8 | 29.5±3.3 | 56.2±4.2 | 36.6±3.6 |
| 5j | 65.7±2.9 | 50.4±3.6 | 45.8±5.5 | 63.4±4.6 | 45.6±4.5 | 67.2±4.4 |
| 5k | 36.4±6.3 | 62.1±6.4 | 76.8±4.8 | 68.4±2.2 | 45.7±3.1 | 53.1±3.7 |
| 5l | 19.6±2.3 | 20.6±1.9 | 35.7±3.4 | 58.2±4.6 | 64.2±2.9 | 60.7±3.6 |
| 5m | 72.5±4.3 | 48.4±3.4 | 32.4±2.1 | 54.0±5.2 | 62.4±3.2 | 43.2±4.2 |
| VP-16 | 8.6±1.4 | 9.1±0.4 | 8.2±1.5 | 8.4±0.6 | 9.8±1.5 | 7.2±1.1 |
| CPT | 7.4±1.5 | 8.7±1.3 | 10.4±1.5 | 7.8±1.4 | 8.5±1.2 | 7.7±1.2 |
| Compd. | IC50/(μmol•L–1) | |||||
|---|---|---|---|---|---|---|
| HeLa | H446 | U-937 | MCF-7 | SK-OV-3 | HT-29 | |
| 5a | 16.5±1.2 | 17.6±3.2 | 12.7±1.1 | 17.7±2.8 | 18.6±2.1 | 19.8±1.9 |
| 5b | 14.5±2.2 | 8.6±1.4 | 10.3±1.9 | 14.2±1.7 | 16.8±1.9 | 14.1±3.4 |
| 5c | 22.4±2.7 | 18.8±2.0 | 11.9±2.4 | 10.5±2.2 | 15.4±1.6 | 12.2±2.3 |
| 5d | 38.4±3.3 | 65.6±3.6 | 72.7±4.1 | 47.5±2.5 | 66.6±2.4 | 72.6±3.9 |
| 5e | 20.5±1.5 | 28.9±1.4 | 21.4±2.1 | 65.1±4.2 | 41.5±2.7 | 25.3±3.2 |
| 5f | 8.7±1.2 | 8.8±2.1 | 14.3±3.1 | 14.9±1.7 | 13.3±2.7 | 21.1±1.6 |
| 5g | 10.6±2.3 | 12.8±1.4 | 10.5±1.7 | 7.4±1.8 | 14.7±3.6 | 12.7±2.3 |
| 5h | 66.7±2.6 | 32.5±1.7 | 45.7±4.5 | 34.6±2.2 | 64.3±3.9 | 22.6±2.4 |
| 5i | 45.2±3.6 | 37.8±3.1 | 35.2±2.8 | 29.5±3.3 | 56.2±4.2 | 36.6±3.6 |
| 5j | 65.7±2.9 | 50.4±3.6 | 45.8±5.5 | 63.4±4.6 | 45.6±4.5 | 67.2±4.4 |
| 5k | 36.4±6.3 | 62.1±6.4 | 76.8±4.8 | 68.4±2.2 | 45.7±3.1 | 53.1±3.7 |
| 5l | 19.6±2.3 | 20.6±1.9 | 35.7±3.4 | 58.2±4.6 | 64.2±2.9 | 60.7±3.6 |
| 5m | 72.5±4.3 | 48.4±3.4 | 32.4±2.1 | 54.0±5.2 | 62.4±3.2 | 43.2±4.2 |
| VP-16 | 8.6±1.4 | 9.1±0.4 | 8.2±1.5 | 8.4±0.6 | 9.8±1.5 | 7.2±1.1 |
| CPT | 7.4±1.5 | 8.7±1.3 | 10.4±1.5 | 7.8±1.4 | 8.5±1.2 | 7.7±1.2 |


| [1] |
Azarova, A. M.; Lyu, Y. L.; Lin, C. P.; Tsai, Y. C.; Lau, J. Y.; Wang, J. C.; Liu, L. F. Natl. Acad. Sci. U. S. A. 2007, 104, 11014.
|
| [2] |
Deweese, J. E.; Osheroff, N. Nucleic Acids Res. 2009, 37, 738.
doi: 10.1093/nar/gkn937 pmid: 19042970 |
| [3] |
Haffner, M. C.; Aryee, M. J.; Toubaji, A.; Esopi, D. M.; Albadine, R.; Gurel, B.; Isaacs, W. B.; Bova, G. S.; Liu, W.; Xu, J.; Meeker, A.K.; Netto, G.; De Marzo, A. M.; Nelson, W. G.; Yegnasubramanian, S. Nat. Genet. 2010, 42, 668.
|
| [4] |
Humphrey, G. R.; Kuethe, J. T. Chem. Rev. 2006, 106, 2875.
pmid: 16836303 |
| [5] |
Horton, D. A.; Bourne, G. T.; Smythe, M. L. Chem. Rev. 2003, 103, 893.
|
| [6] |
Ding, S. E.; Dudley, S.; Plummer, S.; Tang, J.; Newton, R. P.; Brenton, A. G. Phytochemistry 2008, 69, 1555.
doi: 10.1016/j.phytochem.2008.01.026 pmid: 18342344 |
| [7] |
Ahuja, P.; Siddiqui, N. Eur. J. Med. Chem. 2014, 80, 509.
|
| [8] |
Zhang, M. Z.; Mulholl, N.; Beattie, D.; Irwin, D.; Gu, Y. C.; Chen, Q.; Yang, G. F.; Clough, J. Eur. J. Med. Chem. 2013, 63, 22.
|
| [9] |
Zhang, M. Z.; Chen, Q.; Yang, G. F. Eur. J. Med. Chem. 2015, 89, 421.
|
| [10] |
Hu, H. Y.; Wu, J.; Ao, M. T.; Wang, H. R.; Zhou, T. T.; Xue, Y. H.; Qiu, Y. K.; Fang, M. J.; Wu, Z. Chem. Biol. Drug Des. 2016, 88, 766.
|
| [11] |
Hu, H. Y.; Yu, X. D.; Wang, F.; Lin, C. R.; Zeng, J. Z.; Qiu, Y. K.; Fang, M. J.; Wu, Z. Molecules 2016, 21, 1.
|
| [12] |
Hu, H. Y.; Lin, C. R.; Ao, M. T.; Ji, Y. F.; Tang, B. W.; Zhou, X. X.; Fang, M. J.; Zeng, J. Z.; Wu, Z. RSC Adv. 2017, 7, 51640
|
| [13] |
Jiang, B.; Gu, X. H. Bioorg. Med. Chem. 2000, 8, 36
|
| [14] |
Brana, M. F.; Gradillas, A.; Ovalles, A. G.; Lopez, B.; Acero, N.; Llinares, F.; Munoz Mingarro, D. Bioorg. Med. Chem. 2006, 14, 9
|
| [15] |
Shintani, A.; Toume, K.; Rifai, Y.; Arai, M. A.; Ishibashi, M. J. Nat. Prod. 2010, 73, 1711
doi: 10.1021/np1002687 pmid: 20839811 |
| [16] |
Song, Y. L.; Dong, Y. F.; Yang, T.; Zhang, C. C.; Su, L. M.; Huang, X.; Zhang, D. N.; Yang, G. L.; Liu, Y. X. Bioorg. Med. Chem. 2013, 21, 7624
|
| [17] |
Zhang, W. J.; Liu, Z.; Li, S. M.; Yang, T. T.; Zhang, Q. B.; Ma, L.; Tian, X. P.; Zhang, H. B.; Huang, C. G.; Zhang, S.; Ju, J. J.; Shen, Y. M.; Zhang, C. S. Org. Lett. 2012, 14, 3364
|
| [18] |
Liu, Y. P.; Zhao, Y. L.; Feng, T. Cheng, G. G.; Zhang, B. H.; Li, Y.; Cai, X. H.; Luo, X. D. J. Nat. Prod. 2013, 76, 2322
|
| [19] |
Gong, Y.; Firestone, G. L.; Bjeldanes, L. F. Mol. Pharmacol. 2006, 69, 1320.
|
| [20] |
Cho, H. J.; Park, S. Y.; Kim, E. J.; Kim, J. K.; Park, J. H. Y. Mol. Carcinogen. 2011, 50, 100
|
| [21] |
Safe, S.; Papineni, S.; Chintharlapalli, S. Cancer. Lett. 2008, 269, 326
|
| [22] |
Maciejewska, D.; Rasztawicka, M.; Wolska, I.; Anuszewska, E. Z.; Gruber, B. Eur. J. Med. Chem. 2009, 44, 4136.
doi: 10.1016/j.ejmech.2009.05.011 pmid: 19540023 |
| [23] |
Chaniyara, R.; Tala, S.; Chen, C. W.; Zang, X.; Kakadiya, R.; Lin, L. F.; Chen, C. H.; Chien, S. I.; Chou, T. C.; Tsai, T. H. J. Med. Chem. 2013, 56, 1544
doi: 10.1021/jm301788a pmid: 23360284 |
| [24] |
Chen, C. W.; Chen, Y. F.; Chao, S. H.; Tala, S.; Su, T. L.; Lee, T. C. Mol. Cancer Ther. 2013, 12, B259
|
| [25] |
Sun, X. Y.; Feng, S. R.; Dong, J. J.; Feng, J. J.; Liu, Z. M.; Song, Y. L.; Qiao, X. Q. Chin. J. Org. Chem. 2020, 40, 391. (in Chinese)
|
|
(孙晓阳, 冯思冉, 董金娇, 冯佳佳, 刘振明, 宋亚丽, 乔晓强, 有机化学, 2020, 40, 391.)
doi: 10.6023/cjoc201907006 |
|
| [26] |
Song, J. L.; Jones, L. M.; Kishore Kumar, G. D.; Conner, E. S.; Bayeh, L.; Chavarria, G. E.; Charlton-Sevcik, A. K.; Chen, S. E.; Chaplin, D. J.; Trawick, M. L.; Pinney, K. G. Bioorg. Med. Chem. Lett. 2013, 23, 2801.
|
| [27] |
Wei, P.; Liu, J. J.; Ma, J. J.; Yang, G. L. Mod. Appl. Sci. 2010, 12, 136.
|
| [28] |
Liu, Y.; Luo, W.; Sun, L. Drug. Discovery Ther. 2008, 2, 216.
|
| [29] |
Fang, B. L.; Ma, Z. Y.; Yang, G. L.; Wang, G.; Tian, Wei.; Li, L. B. Int. J. Chem. 2010, 2, 143.
|
| [30] |
Han, X. Y.; Li, S. B.; Liang, G. C.; Song, Y. L. Acta Pharm. Sin. 2017, 52, 113. (in Chinese)
|
|
(韩晓燕, 李生彬, 梁国超, 宋亚丽, 药学学报, 2017, 52, 113.)
|
|
| [31] |
Han, Q.; Pabba, P. K.; Barbosa, J.; Mabon, R.; Healy, J. P.; Gardyan, M. W.; Terranova, K. M.; Brommage, R.; Thompson, A.Y.; Schmidt, J. M.; Wilson, A. G.; Xu, X. L.; Tarverjr, J. E.; Carson, K. G. Bioorg. Med. Chem. Lett. 2016, 26, 1103.
|
| [32] |
Wang, D. J.; Hou, Z.; Xu, H.; An, R.; Su, X.; Guo, C. Bioorg. Med. Chem. Lett. 2018, 28, 3574.
|
| [33] |
Yin, L. J.; Li, C. Q.; Wu, X. X.; Xu, G. S.; Li, Z. Y.; Shen, Y. M. Chin. J. Org. Chem. 2022, 42, 293. (in Chinese)
|
|
(尹丽君, 李超群, 吴晓霞, 徐广森, 李志颖, 沈月毛, 有机化学, 2022, 42, 293.)
doi: 10.6023/cjoc202105008 |
|
| [34] |
Han, X. Y. M.S. Thesis, Hebei University, Baoding, 2017. (in Chinese)
|
|
(韩晓燕, 硕士论文, 河北大学, 保定, 2017.)
|
|
| [35] |
Mosmann, T. J. Immunol. Methods 1983, 65, 55.
doi: 10.1016/0022-1759(83)90303-4 pmid: 6606682 |
| [1] | Liqing Qin, Guishan Lin, Wengui Duan, Yucheng Cui, Maofang Yang, Fangyao Li, Dianpeng Li. Synthesis, Antiproliferative Activity, 3D-QSAR and Molecular Docking Study of Novel Longifolene-Derived Tetraline Fused N-Acyl-pyrazole Compounds [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1967-1977. |
| [2] | Yiming Hu, Jiayu Xu, Min Tang, Yawen Liu, Liping Guan, Qinghao Jin. Design, Synthesis and Biological Activity Studies of 2-(1,3-Dioxoiso-indolin-2-yl)-N-phenethylacetamide and 2-(3,4-Dihydroisoquinolin-1-yl)isoindole-1,3-dione as Monoamine Oxidase (MAO) and Cholinesterase (ChE) Inhibitors [J]. Chinese Journal of Organic Chemistry, 2024, 44(6): 1907-1919. |
| [3] | Hongyan Pei, Jialin Ye, Feng Wang, Dongdong Liu, Yukui Yu, Jing Zhang, Lixin Zhang. Design, Synthesis and Herbicidal Activity of Novel Uracil Compounds Containing Piperidine Moiety [J]. Chinese Journal of Organic Chemistry, 2024, 44(5): 1592-1605. |
| [4] | Simin Wu, Jiaxin Tang, Yujia Zhou, Xuetao Xu, Haoxing Zhang, Shaohua Wang. α-Glucosidase Inhibition Research of Derivatives Based on 2β-Acetoxyferruginol Scaffold Excluding Acetic Acid Group [J]. Chinese Journal of Organic Chemistry, 2024, 44(2): 613-621. |
| [5] | Bozhen Wang, Jie Zhang, Chunhui Nian, Mingming Jin, Miaomiao Kong, Wulan Li, Wenfei He, Jianzhang Wu. Synthesis and Antitumor Activity of 3,4-Dichlorophenyl Amides [J]. Chinese Journal of Organic Chemistry, 2024, 44(1): 232-241. |
| [6] | Feng Wang, Yu Chen, Hongyan Pei, Jing Zhang, Lixin Zhang. Design, Synthesis and Antifungal Activities of Novel 1,2,4-Oxadiazole Derivatives Containing Piperidine [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2826-2836. |
| [7] | Min Liu, Dongyan Yang, Yumei Xiao, Wangcang Su, Fenghai Zhao, Qin Zhaohai .. Synthesis and Bioactivities of 5-Nitroimino-[1,4-2H]-1,2,4-triazolines as Olefin-Imidacloprid Mimics [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2790-2799. |
| [8] | Panxing Pang, Rong Ning, Chuang Zhu, Wenjie Huang, Xianli Ma, Caina Jiang, Fangyao Li, Xiaoqun Zhou. Synthesis and in Vitro Antitumor Activity of Matrine Semicarbazide Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(6): 2126-2135. |
| [9] | Kanghui Duan, Junlong Tang, Wanqing Wu. Recent Advances in the Synthesis of Fused Heterocyclic Compounds and Their Antitumor Activities [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 826-854. |
| [10] | Chujie Liao, Hongyao Ruan, Junfeng Jiang, Lun Luo, Yanggen Hu. Synthesis and Activity Evaluation of 3-Aryl-2-imino-benzo[e][1,3]-oxazin-4-ol Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 763-770. |
| [11] | Huan Xu, Hongfei Wu, Xiaoming Zhang, Xingxing Lu, Tengda Sun, Yue Qi, Yufan Lin, Xinling Yang, Li Zhang, Yun Ling. Design, Synthesis and Bioactivity of Sulfonyl Hydrazides and Hydrazides Containing Fragment 1,2,3,4-Tetrahydroisoquinoline [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 725-733. |
| [12] | Weiqin Liu, Lihui Shao, Chengpeng Li, Yayu Zou, Haitao Long, Yan Li, Qiangsheng Ge, Zhenchao Wang, Guiping Ouyang. Synthesis and Antitumor Activity of 3-Hydrazone Quinazolinone Derivatives [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 214-222. |
| [13] | Changxing Sun, Fuhao Zhang, Huan Zhang, Penghui Li, Lin Jiang. Design, Synthesis, Fungicidal Activity and Molecular Docking Study of Novel 2-(1-Methyl-1H-pyrazol-4-yl)pyrimidine-4-carboxamides [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 229-235. |
| [14] | Guangping Liang, Wei Wang, Xuxiu Zhu, Guangyan Liang, Jun Yang, Daoping Wang. Synthesis and in Vitro Anti-tumor Activity of Novel Spliced Compounds of Zidovudine and 4-Anilinoquinazolines [J]. Chinese Journal of Organic Chemistry, 2022, 42(9): 2793-2805. |
| [15] | Dongyan Hu, Guangtian Han, Xi'an Li, Huazhong Ren, Lirong Yue, Li Guo, Jiafu Feng. Synthesis and Evaluation in vitro of Novel Harmine Derivatives as Anticancer Activity Agents [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1863-1871. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||