

Chinese Journal of Organic Chemistry >
Research Progress of Fluorescence Probes Constructed by Cyclodextrin Derivatives and Inclusion Complexes
Received date: 2021-08-16
Revised date: 2021-10-21
Online published: 2021-11-10
Supported by
Natural Science Foundation of Liaoning Province(2020-MS-289); Program for Distinguished Professor of Liaoning Province
Cyclodextrins are a kind of the most important host compounds in supramolecular chemistry, and have been widely used in many fields such as drug release, chemical sensing, enantiomer separation and new materials. Because cyclodextrins have a barrel-shaped structure that is hydrophilic on the outside and hydrophobic on the inside, receptors with recognition functions or fluorescent dyes can be inserted into the cavity of cyclodextrins, which can realize the recognition of target molecules by chelation or displacement. Therefore, construction of fluorescent probes based on cyclodextrins derivatives and inclusion complexes has been received great attention. In this paper, the applications of fluorescent probes that designed and synthesized based on cyclodextrins in the detection of metal ions, anions and molecules are summarized, the recognition performance and mechanisms are described, and it is expected to provide theoretical basis for the application of cyclodextrin derivatives and inclusion complexes in the field of fluorescence detection.
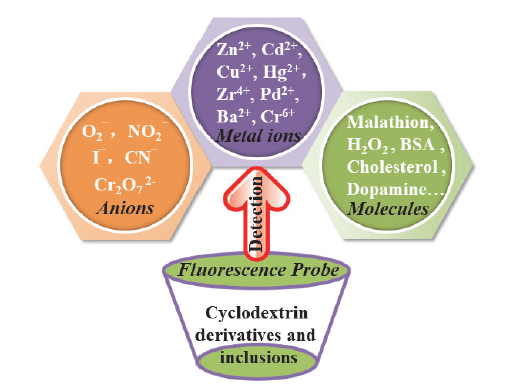
Key words: cyclodextrins; inclusion; fluorescent probe; recognition; progress
Yuqing He , Lin Chen , Ruili He , Keli Zhong , Lijun Tang . Research Progress of Fluorescence Probes Constructed by Cyclodextrin Derivatives and Inclusion Complexes[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 785 -795 . DOI: 10.6023/cjoc202108024
| [1] | Tang, L.; Xia, J.; Zhong, K.; Tang, Y.; Gao, X.; Li, J. Dyes Pigm. 2020, 178, 108379. |
| [2] | Peng, R.; Xu, Y.; Cao, Q. Chin. Chem. Lett. 2018, 29, 1465. |
| [3] | Luo, X.; Li, J.; Zhao, J.; Gu, L.; Qian, X.; Yang, Y. Chin. Chem. Lett. 2019, 30, 839. |
| [4] | He, L.; Dong, B.; Liu, Y.; Lin, W. Chem. Soc. Rev. 2016, 45, 6449. |
| [5] | Tang, B.; Du, X.; Qin, A.; Wang, J. Chin. Sci. Bull. 2020, 65, 1428. |
| [6] | Wang, D.; Fan, X.; Sun, S.; Du, S.; Li, H.; Zhu, J.; Tang, Y.; Chang, M.; Xu, Y. Sens Actuators, B 2018, 264, 304. |
| [7] | Ege, Z. R.; Akan, A.; Oktar, F. N.; Lin, C. C.; Kuruca, D. S.; Karademir, B.; Sahin, Y. M.; Erdemir, G.; Gunduz, O. J. Biomed. Mater. Res. B, Appl. Biomater. 2019, 108, 538. |
| [8] | Kang, Y. F.; Niu, L. Y.; Yang, Q. Z. Chin. Chem. Lett. 2019, 30, 1791. |
| [9] | Gao, W.; Wang, W.; Yao, S.; Wu, S.; Zhang, H.; Zhang, J.; Jing, F.; Mao, H.; Jin, Q.; Cong, H. Anal. Chim. Acta 2017, 958, 77. |
| [10] | Zhu, Z.; Liu, W.; Cheng, L.; Li, Z.; Xi, Z.; Yi, L. Tetrahedron Lett. 2015, 56, 3909. |
| [11] | Tang, L.; Zhou, L.; Yan, X.; Zhong, K.; Gao, X.; Liu, X.; Li, J. Dyes Pigm. 2020, 182, 108644. |
| [12] | Zhong, K.; He, Y.; Deng, L.; Yan, X.; Li, X.; Tang, Y.; Hou, S.; Tang, L. Anal. Chim. Acta 2020, 1127, 49. |
| [13] | Liu, X.; Li, N.; Li, M.; Chen, H.; Zhang, N.; Wang, Y.; Zheng, K. Coord. Chem. Rev. 2020, 404. |
| [14] | Yu, L.; Qiao, Y.; Miao, L.; He, Y.; Zhou, Y. Chin. Chem. Lett. 2018, 29, 1545. |
| [15] | Tian, B.; Liu, Y.; Liu, J. Carbohydr. Polym. 2020, 116871. |
| [16] | Szente, L.; Szejtli, J. Trends Food Sci. Technol. 2004, 15, 137. |
| [17] | Zhang, Y.; Liu, Y. Chin. J. Org. Chem. 2020, 40, 3802. (in Chinese) |
| [17] | (张依, 刘育, 有机化学, 2020, 40, 3802.) |
| [18] | Adeoye, O.; Cabral-Marques, H. Int. J. Pharm. 2017, 531, 521. |
| [19] | Tian, B.; Hua, S.; Tian, Y.; Liu, J. Environ. Sci. Pollut. Res. 2020, 28, 1317. |
| [20] | Zhang, Y. M.; Liu, Y. H.; Liu, Y. Adv. Mater. 2020, 32, 1806158. |
| [21] | Tian, B.; Xiao, D.; Hei, T.; Ping, R.; Hua, S.; Liu, J. Polym. Int. 2020, 69, 597. |
| [22] | Luo, X.; Gu, L.; Qian, X.; Yang, Y. Chem. Commun. 2020, 56, 9067. |
| [23] | Tian, X.; Zuo, M.; Niu, P.; Wang, K.; Hu, X. Chin. J. Org. Chem. 2020, 40, 1823. (in Chinese) |
| [23] | (田雪琪, 左旻瓒, 牛蓬勃, 王开亚, 胡晓玉, 有机化学, 2020, 40, 1823.) |
| [24] | Liu, Z.; Dai, X.; Sun, Y.; Liu, Y. Aggregate 2020, 1, 31. |
| [25] | Zhang, N.; Chen, Y.; Yu, M.; Liu, Y. Chem. Asian J. 2009, 4, 1697. |
| [26] | Zhang, L.; Hu, W.; Yu, L.; Wang, Y. Chem. Commun. 2015, 51, 4298. |
| [27] | Khan, R. I.; Pitchumani, K. RSC Adv. 2016, 6, 20269. |
| [28] | Yang, S. L.; Jiang, W. N.; Tang, Y.; Xu, L.; Gao, B.-H.; Xu, H.-J. Chin. J. Anal. Chem. 2019, 47, e19059. |
| [29] | Maniyazagan, M.; Rameshwaran, C.; Mariadasse, R.; Jeyakanthan, J.; Premkumar, K.; Stalin, T. Sens. Actuators, B 2017, 242, 1227. |
| [30] | Prabu, S.; Mohamad, S. J. Mol. Struct. 2020, 1204, 127528. |
| [31] | Meng, H. M.; Fu, T.; Zhang, X. B.; Wang, N. N.; Tan, W.; Shen, G. L.; Yu, R. Q. Anal. Chem. 2012, 84, 2124. |
| [32] | Maniyazagan, M.; Mohandoss, S.; Sivakumar, K.; Stalin, T. Spectrochim. Acta, Part A 2014, 133, 73. |
| [33] | Sivakumar, K.; Parameswari, M.; Stalin, T. J. Carbohydr. Chem. 2016, 35, 118. |
| [34] | Wang, J.; Qiu, F.; Wu, H.; Li, X.; Zhang, T.; Niu, X.; Yang, D.; Pan, J.; Xu, J. Spectrochim. Acta, Part A 2017, 179, 163. |
| [35] | Teranishi, K.; Nishiguchi, T. Anal. Biochem. 2004, 325, 185. |
| [36] | Gao, F.; Zhang, L.; Wang, L.; She, S.; Zhu, C. Anal. Chim. Acta 2005, 533, 25. |
| [37] | Ren, S. H.; Liu, S. G.; Ling, Y.; Li, N. B.; Luo, H. Q. Spectrochim. Acta, Part A 2019, 212, 199. |
| [38] | Li, Q.; Zhang, Y.; Jin, Y.; Yang, Q.; Du, J.; Li, Y. RSC Adv. 2015, 5, 68815. |
| [39] | Mohandoss, S.; Sivakamavalli, J.; Vaseeharan, B.; Stalin, T. Sens. Actuators, B 2016, 234, 300. |
| [40] | Wang, L. Y.; Dong, L. Y.; Chen, L.; Fan, Y. B.; Wu, J.; Wang, X. F.; Xie, M. X. New J. Chem. 2015, 39, 555. |
| [41] | Tan, S. Y.; Teh, C.; Ang, C. Y.; Li, M.; Li, P.; Korzh, V.; Zhao, Y. Nanoscale 2017, 9, 2253. |
| [42] | Sun, Q.; Fang, S.; Fang, Y.; Qian, Z.; Feng, H. Talanta 2017, 167, 513. |
| [43] | Wang, M.; Su, K.; Cao, J.; She, Y.; Abd, El-Aty, A. M.; Hacimuftuoglu, A.; Wang, J.; Ya, n M.; Hong, S.; Lao, S.; Wang, Y. Talanta 2019, 192, 295. |
| [44] | Lu, X.; Fan, Z. Spectrochim. Acta, Part A 2019, 216, 342. |
| [45] | Liu, L.; Yi, G.; Yang, L.; Li, K.; Dong, G.; Sun, Y.; Zhang, H. Carbohydr. Polym. 2020, 116367. |
| [46] | Halawa, M. I.; Wu, F.; Fereja, T. H.; Lou, B.; Xu, G. Sens. Actuators, B 2018, 254, 1017. |
| [47] | Zhu, X.; Hu, Y.; Gong, A. Anal. Chim. Acta 2007, 592, 24. |
| [48] | Zhu, X.; Sun, J.; Hu, Y. Anal. Chim. Acta 2007, 596, 298. |
| [49] | Patra, D. Biosens. Bioelectron. 2010, 25, 1149. |
| [50] | Wu, X.; Lin, L. R.; Huang, Y. J.; Li, Z.; Jiang, Y. B. Chem. Commun. 2012, 48, 4362. |
| [51] | Wang, X.; Zeng, H.; Zhao, L.; Lin, J.-M. Anal. Chim. Acta 2006, 556, 313. |
| [52] | Zhu, X.; Xu, S. Spectrochim. Acta, Part A 2010, 77, 566. |
| [53] | Paul, B. K.; Guchhait, N. J. Colloid Interface Sci. 2011, 353, 237. |
| [54] | Gao, F.; Shang, Y. J.; Zhang, L.; She, S. K.; Wang, L. Anal. Lett. 2004, 37, 1285. |
| [55] | Wang, L.; Bian, G.; Wang, L.; Dong, L.; Chen, H.; Xia, T. Spectrochim. Acta, Part A 2005, 61, 1201. |
| [56] | Liu, P.; Sun, S.; Guo, X.; Yang, X.; Huang, J.; Wang, K.; Wang, Q.; Liu, J.; He, L. Anal. Chem. 2015, 87, 2665. |
| [57] | Huang, H.; Yang, X.; Wang, K.; Wang, Q.; Guo, Q.; Huang, J.; Liu, J.; Guo, X.; Li, W.; He, L. Talanta 2015, 144, 529. |
| [58] | Song, C.; Li, B.; Yang, X.; Wang, K.; Wang, Q.; Liu, J.; Huang, J. Analyst 2016, 142, 224. |
| [59] | Zhao, X.; Chen, Y.; Dai, X.; Zhou, W.; Li, J.; Liu, Y. Adv. Photonics Res. 2020, 1, 2000007. |
| [60] | Yu, J.; Chen, Y.; Li, J. J.; Liu, Y. J. Mater. Chem. C 2017, 5, 799. |
/
| 〈 |
|
〉 |