

Chinese Journal of Organic Chemistry >
Research Progress of Peroxygenase-Catalyzed Reactions Driven by in-situ Generation of H2O2
Received date: 2021-08-28
Revised date: 2021-09-30
Online published: 2021-11-17
Supported by
National Natural Science Foundation of China(32171253)
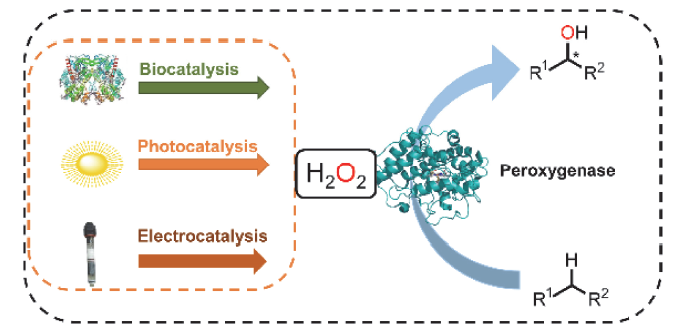
Selective catalytic oxygen functionalization reactions of C—H bonds are a very challenging class of reactions in organic chemistry. Compared with tranditional metal complex catalysts, enzymes exhibit certain advantages in terms of selectivity and activity. Amongest them, peroxygenases are able to catalyze the oxidative functionalization of C—H bonds by direct use of H2O2 as a co-substrate. Peroxygenases combine the catalytic versatility of P450 monooxygenases without relying on coenzymes and their regenerative systems for cofactors. Therefore, peroxygenases have attracted considerable attention in organic synthesis. However, a bottleneck in the practical application of peroxygenase is that, like all heme-dependent enzymes, peroxygenase is sensitive to H2O2, and higher concentration of H2O2 in the reaction system will cause oxidative decomposition of ferrous heme, resulting in the enzyme inactivation. To overcome this, regulating the concentration of H2O2 in the reaction is particularly important for the efficient use of peroxygenase. This paper outlines a variety of methods used for in situ generation of H2O2 to drive peroxygenases reported in recent years. The pros and cons of biocatalysis, photocatalysis and electrocatalysis used for high atom-efficient H2O2 generation are comprehensively analyzed. This review paper is expected to provide a reference for promoting the application of peroxygenases in organic synthesis.
Kexin Li , Qingyuan Yang , Pengpeng Zhang , Wuyuan Zhang . Research Progress of Peroxygenase-Catalyzed Reactions Driven by in-situ Generation of H2O2[J]. Chinese Journal of Organic Chemistry, 2022 , 42(3) : 732 -741 . DOI: 10.6023/cjoc202108052
| [1] | Dong, J.-J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C. E.; Pasic, M.; Schmidt, S.; Wang, Y.-H.; Younes, S.; Zhang, W.-Y. Angew. Chem., Int. Ed. 2018, 57, 9238. |
| [2] | Zeng, Z.-G.; Sang, X.-K.; Yuan, B.; Wu, M.-H.; Zhang, W.-Y. Chin. J. Org. Chem. 2021, 41, 959. (in Chinese) |
| [2] | (曾志刚, 桑贤轲, 袁波, 吴鸣虎, 张武元, 有机化学, 2021, 41, 959.) |
| [3] | Bormann, S.; Baraibar, A. G.; Ni, Y.; Holtmann, D.; Hollmann, F. Catal. Sci. Technol. 2015, 5, 2038. |
| [4] | Ullrich, R.; Nüske, J.; Scheibner, K.; Spantzel, J.; Hofrichter, M. Appl. Environ. Microbiol. 2004, 70, 4575. |
| [5] | Hofrichter, M.; Ullrich, R. Appl. Microbiol. Biotechnol. 2006, 71, 276. |
| [6] | Zhang, W.-Y.; Hollmann, F. Synthesis of Vinyl Polymers via Enzymatic Oxidative Polymerisation, Springer Nature Singapore Pte Ltd, Singapore, 2019, p. 343. |
| [7] | Peter, S.; Kinne, M.; Wang, X.-S.; Ullrich, R.; Kayser, G.; Groves, J. T.; Hofrichter, M. FEBS J. 2011, 278, 3667. |
| [8] | Lucas, F.; Babot, E. D.; Cañellas, M.; del Río, J. C.; Kalum, L.; Ullrich, R.; Hofrichter, M.; Guallar, V.; Martínez, A. T.; Gutiérrez, A. Catal. Sci. Technol. 2016, 6, 288. |
| [9] | Kinne, M.; Ullrich, R.; Hammel, K. E.; Scheibner, K.; Hofrichter, M. Tetrahedron Lett. 2008, 49, 5950. |
| [10] | de Santons, P. G.; Cañellas, M.; Tieves, F.; Younes, S. H. H.; Molina-Espeja, P.; Hofrichter, M.; Hollmann, F.; Guallar, V.; Alcalde, M. ACS Catal. 2018, 8, 4789. |
| [11] | Molina-Espeja, P.; Cañellas, M.; Plou, F. J.; Hofrichter, M.; Lucas, F.; Guallar, V.; Alcalde, M. ChemBioChem 2016, 17, 341. |
| [12] | Thiel, D.; Doknić, D.; Deska, J. Nat. Commun. 2014, 5, 5278. |
| [13] | Valderrama, B.; Ayala, M.; Vazquez-Duhalt, R. Chem. Biol. 2002, 9, 555. |
| [14] | van de Velde, F.; Lourenco, N. D.; Bakker, M.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 69, 286. |
| [15] | Bakker, M.; van de Velde, F.; van Rantwijk, F.; Sheldon, R. A. Biotechnol. Bioeng. 2000, 70, 342. |
| [16] | Okrasa, K.; Guibé-Jampel, E.; Therisod, M. J. Chem. Soc., Perkin Trans. 1 2000, 7, 1077. |
| [17] | Ribitsch, D.; Karl, W.; Wehrschütz-Sigl, E.; Tutz, S.; Remler, P.; Weber, H. J.; Gruber, K.; Stehr, R.; Bessler, C.; Hoven, N.; Sauter, K.; Schwab, H. Appl. Microbiol. Biotechnol. 2009, 81, 875. |
| [18] | Okrasa, K.; Falcimaigne, A.; Guibé-Jampel, E.; Therisod, M. Tetrahedron: Asymmetry 2002, 13, 519. |
| [19] | Pezzotti, F.; Okrasa, K.; Therisod, M. Tetrahedron: Asymmetry 2005, 16, 2681. |
| [20] | Pezzotti, F.; Therisod, M. Tetrahedron: Asymmetry 2007, 18, 701. |
| [21] | Rocha-Martin, J.; Velasco-Lozano, S.; Guisán, J. M.; López- Gallego, F. Green Chem. 2014, 16, 303. |
| [22] | Jung, D.; Streb, C.; Hartmann, M. Microporous Mesoporous Mater. 2008, 113, 523. |
| [23] | Ni, Y.; Fernández-Fueyo, E.; Baraibar, A. G.; Ullrich, R.; Hofrichter, M.; Yanase, H.; Alcalde, M.; van Berkel, W. J. H.; Hollmann, F. Angew. Chem., Int. Ed. 2015, 55, 798. |
| [24] | Chang, A.; Scheer, M.; Grote, A.; Schomburg, I.; Schomburg, D. Nucleic Acids Res. 2009, 37, D588. |
| [25] | Pesic, M; Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Alcalde, M.; Hollamnn, F. Z. Naturforsch., C: J. Biosci. 2018, 74, 100. |
| [26] | Tieves, F.; Willot, S. J. P.; van Schie, M. M. C. H.; Rauch, M. C. R.; Younes, S. H. H.; Zhang, W.-Y.; Dong, J.-J.; de Santos, P. G.; Robbins, J. M.; Bommarius, B.; Alcalde, M.; Bommarius, A. S.; Hollmann, F. Angew. Chem., Int. Ed. 2019, 58, 7873. |
| [27] | Willot, S. J. P.; Hoang, M. D.; Paul, C. E.; Alcalde, M.; Arends, I. W. C. E.; Bommarius, A. S.; Bommarius, B.; Hollmann, F. ChemCatChem 2020, 12, 2713. |
| [28] | Li, Y.-Y.; Yuan, B.; Sun, Z.-T.; Zhang, W.-Y. Green Synth. Catal. 2021, 2, 267. |
| [29] | Perez, D. I.; Grau, M. M.; Arends, I. W. C. E.; Hollmann, F. Chem. Commun. 2009, 41, 6848. |
| [30] | Churakova, E.; Kluge, M.; Ullrich, R.; Arends, I.; Hofrichter, M.; Hollmann, F. Angew. Chem., Int. Ed. 2011, 50, 10716. |
| [31] | Churakova, E.; Arends, I. W. C. E.; Hollmann, F. ChemCatChem 2013, 5, 565. |
| [32] | Zhang, W.-Y.; Burek, B. O.; Fernández-Fueyo, E.; Alcalde, M.; Bloh, J. Z.; Hollmann, F. Angew. Chem. 2017, 56, 15451. |
| [33] | Zhang, W.-Y.; Fernández-Fueyo, E.; Ni, Y.; van Schie, M.; Gacs, J.; Renirie, R.; Wever, R.; Mutti, F. G.; Rother, D. R.; Alcalde, M.; Hollmann, F. Nat. Catal. 2018, 1, 55. |
| [34] | Teranishi, M.; Hoshino, R.; Naya, S.; Tada, H. Angew. Chem. 2016, 128, 12965. |
| [35] | van Schie, M. M. C. H.; Zhang, W.-Y.; Tieves, F.; Choi, D. S.; Park, C. B.; Burek, B. O.; Bloh, J. Z.; Arends, I. W. C. E.; Paul, C. E.; Alcalde, M.; Hollmann, F. ACS Catal. 2019, 9, 7409. |
| [36] | Willot, S. J. P.; Fernández-Fueyo, E.; Tieves, F.; Pesic, M.; Alcalde, M.; Arends, I. W. C. E.; Park, C. B.; Hollmann, F. ACS Catal. 2019, 9, 890. |
| [37] | Yuan, B.; Mahor, D.; Fei, Q.; Wever, R.; Alcalde, M.; Zhang, W.-Y.; Hollmann, F. ACS Catal. 2020, 10, 8277. |
| [38] | Zhang, W.-Y.; Liu, H.-H.; van Schie, M. M. C. H.; Hagedoorn, P. L.; Alcalde, M.; Denkova, A. G.; Djanashvili, K.; Hollmann, F. ACS Catal. 2020, 10, 14195. |
| [39] | Lvtz, S.; Steckhan, E.; Liese, A. Electrochem. Commun. 2004, 6, 583. |
| [40] | Kohlmann, C.; Lvtz, S. Eng. Life Sci. 2006, 6, 170. |
| [41] | Lvtz, S.; Vuorilehto, K.; Liese, A. Biotechnol. Bioeng. 2007, 98, 525. |
| [42] | Horst, A. E. M.; Mangold, K. M.; Holtmann, D. Biotechnol. Bioeng. 2016, 113, 260. |
| [43] | Krieg, T.; Hvttmann, S.; Mangold, K. M.; Schrader, J.; Holtmann, D. Green Chem. 2011, 13, 2686. |
| [44] | Holtmann, D.; Krieg, T.; Getrey, L.; Schrader, J. Catal. Commun. 2014, 51, 82. |
| [45] | Horst, A. E. W.; Bormann, S.; Meyer, J.; Steinhagen, M.; Ludwig, R.; Drews, A.; Ansorge-Schumacher, M.; Holtmann, D. J. Mol. Catal. B: Enzym. 2016, 133, S137. |
| [46] | Choi, D. S.; Ni, Y.; Fernández-Fueyo, E.; Lee, M.; Hollmann, F.; Park, C. B. ACS Catal. 2017, 7, 1563. |
| [47] | Kim, H. W.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J.-H.; Yang, P.-D.; McCloskey, B. D. Nat. Catal. 2018, 1, 282. |
| [48] | Bormann, S.; van Schie, M. M. C. H.; De Almeida, T. P.; Zhang, W.-Y.; Stöckl, M.; Ulber, R.; Hollmann, F.; Holtmann, D. ChemSusChem 2019, 12, 4759. |
| [49] | Karmee, S. K.; Roosen, C.; Kohlmann, C.; Lvtz, S.; Greiner, L.; Leitner, W. Green Chem. 2009, 11, 1052. |
| [50] | Edwards, J. K.; Freakley, S. J.; Carley, A. F.; Kiely, C. J.; Hutchings, G. J. Acc. Chem. Res. 2014, 47, 845. |
| [51] | Freakley, S. J.; Kochius, S.; van Marwijk, J.; Fenner, C.; Lewis, R. J.; Baldenius, K.; Marais, S. S.; Opperman, D. J.; Harrison, S. T. L.; Alcalde, M.; Smit, M. S.; Hutchings, G. J. Nat. Commun. 2019, 10, 1. |
| [52] | Yoon, J.; Kim, J.; Tieves, F.; Zhang, W.-Y.; Alcalde, M.; Hollmann, F.; Park, C. B. ACS Catal. 2020, 10, 5236. |
/
| 〈 |
|
〉 |