

Chinese Journal of Organic Chemistry >
Tetraethylenepentamine Functionalized Phenolic Resin as Highly Active Acid-Base Bifunctional Catalyst for Knoevenagel Condensation Reaction
Received date: 2021-09-21
Revised date: 2021-11-06
Online published: 2021-12-08
Supported by
Natural Science Foundation of Hebei Province(H2020209288)
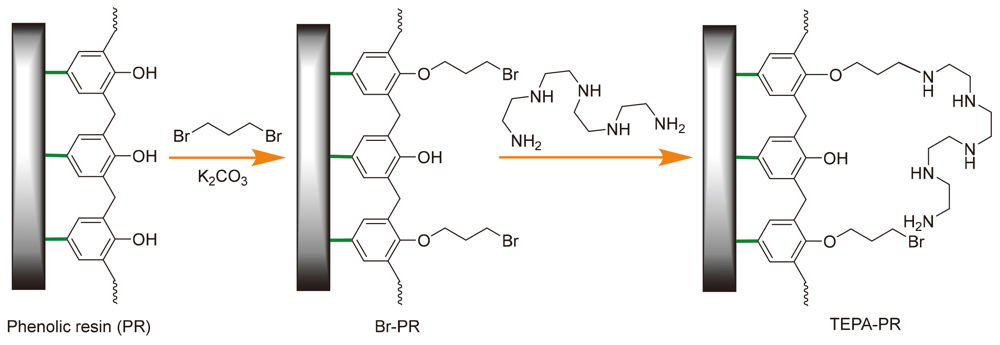
The tetraethylenepentamine functionalized phenolic resin (TEPA-PR) for Knoevenagel condensation reaction has been prepared by aminating a commercially available phenolic resin (PR) and tetraethylenepentamine. The TEPA-PR was characterized by Fourier-transfer infrared spectroscopy (FTIR), X-ray diffraction spectra (XRD) and elemental analysis (EA). The TEPA-PR can efficiently catalyze Knoevenagel condensation reaction of aromatic aldehydes with high yields of a wide range of products. Furthermore, the reaction can be easily carried out in different solvents of different polarity. The novel developed resin catalyst shows good reusability (5 times) without significant decrease of catalytic activity.
JIan Xiao , Zhiying Wu , Ziyi Chen , Pengfei Zhao . Tetraethylenepentamine Functionalized Phenolic Resin as Highly Active Acid-Base Bifunctional Catalyst for Knoevenagel Condensation Reaction[J]. Chinese Journal of Organic Chemistry, 2022 , 42(4) : 1179 -1187 . DOI: 10.6023/cjoc202109031
| [1] | (a) Varga G.; Kukovecz Á.; Kónya Z.; Sipos P.; Pálinkó I. J. Catal. 2020, 381, 308. |
| [1] | (b) Moteki T.; Koga Y.; Ogura M. J. Catal. 2019, 378, 131. |
| [1] | (c) Zacuto M. J. J. Org. Chem. 2019, 84, 6465. |
| [1] | (d) Li J. P. H.; Kennedy E. M.; Adesina A. A.; Stockenhuber M. J. Catal. 2019, 369, 157. |
| [1] | (e) Jia Y.; Fang Y.; Zhang Y.; Miras H. N.; Song Y. Chem.-Eur. J. 2015, 21, 14862. |
| [1] | (f) Garrabou X.; Wicky B. I. M.; Hilvert D. J. Am. Chem. Soc. 2016, 138, 6972. |
| [1] | (g) Franconetti A.; Domínguez-Rodríguez P.; Lara-García D.; Prado-Gotor R.; Cabrera-Escribano F. Appl. Catal. A: Gen. 2016, 517, 176. |
| [1] | (h) Ezugwu C. I.; Mousavi B.; Asraf M. A.; Luo Z.; Verpoort F. J. Catal. 2016, 344, 445. |
| [2] | (a) Kumar N. S.; Reddy M. S.; S. Kumar T. S.; Bheeram V. R.; Mukkamala S. B.; Rao L. C. ChemistrySelect 2019, 4, 1188. |
| [2] | (b) Gu X.; Tang Y.; Zhang X.; Luo Z.; Lu H. New J. Chem. 2016, 40, 6580. |
| [2] | (c) Pandey R.; Singh D.; Thakur N.; Raj K. K. ACS Omega 2021, 6, 13240. |
| [2] | (d) Grass J.; Klühspies K.; Reiprich B.; Schwieger W.; Inayat A. Catalysts 2021, 11, 474. |
| [2] | (e) Das A.; Anbu N.; SK M. Dalton Trans. 2019, 4, 17371. |
| [2] | (f) Zhang X.; Zhang R.; Jin Y.; Li T. J. Solid State Chem. 2019, 278, 120927. |
| [2] | (g) Guo C. Y.; Zhang Y. H.; Zhang L.; Zhang Y.; Wang J. D. CrystEngComm 2018, 20, 5327. |
| [3] | (a) Das A.; Anbu N.; Dhakshinamoorthy A.; Biswas S. Microporous Mesoporous Mater. 2019, 284, 459. |
| [3] | (b) Gao M.; Qi M.; Liu L.; Han Z. Chem. Commun. 2019, 55, 6377. |
| [4] | (a) Zhang X. Y.; He X. Y.; Zhao S. H. Green Chem Lett. Rev. 2021, 14, 85. |
| [4] | (b) Jiang Y.; Jiang F.; Liao X.; Lai S.; Wang S.; Xiong X.; Zheng J.; Liu Y. J. Porous Mater. 2020, 27, 779. |
| [5] | (a) Appaturi J. N.; Pulingam T.; Rajabathar J. R.; Khoerunnisa F.; Ling T. C.; Tan S. H.; Ng E. Microporous Mesoporous Mater. 2021, 320, 111091. |
| [5] | (b) Li R. J.; Chen C.; Hu L. Y.; ChemistrySelect 2020, 5, 14578. |
| [6] | (a) Jaenicke S.; Chuah G. K.; Lin X. H.; Hu X. C. Micro¬porous Mesoporous Mater. 2000, 35-36, 143. |
| [7] | (a) Qian B.; Wang F.; Li D.; Li Y. Zhang B.; Zhu J. New J. Chem. 2020, 44, 5995. |
| [7] | (b) Zhu J.; Wang F.; Li D.; Zhai J.; Liu P.; Zhang W.; Li Y.; Catal. Lett. 2020, 150, 1909. |
| [7] | (c) Xue B.; Liu X.; Liu N.; Li Y. Res. Chem. Intermed. 2018, 44, 1523. |
| [8] | (a) Hajizadeh F.; Maleki B.; Zonoz F. M.; Amiri A. J. Iran. Chem. Soc. 2021, 18, 793. |
| [8] | (b) Hu Y.; Zhang J.; Wang Z.; Huo H.; Jiang Y.; Xu X.; Lin K. ACS Appl. Mater. Inter. 2020, 12, 36159. |
| [9] | (a) Zhang X.; He X.; Zhao S. Green Chem. Lett. Rev. 2021, 14, 85. |
| [9] | (b) Gilanizadeh M.; Zeynizadeh B. Can. J. Chem. 2021, 99, 531. |
| [10] | (a) Yao C.; Zhou S.; Kang X.; Zhao Y.; Yan R.; Zhang Y.; Wen L. Inorg. Chem. 2018, 57, 11157. |
| [10] | (b) Sarmah B.; Srivastava R. Mol. Catal. 2017, 427, 62. |
| [10] | (c) Zhang L.; Wang H.; Shen W.; Qin Z.; Wang J.; Fan W. J. Catal. 2016, 344, 293. |
| [11] | Xiao J.; Wang L.; Ran J.; Zhao J.; Ma N.; Tao M.; Zhang W. J. Cleaner Prod. 2020, 274, 122473. |
| [12] | Laha B.; Khullar S.; Gogia A.; Mandal S. K. Dalton Trans. 2020, 49, 12298. |
| [13] | Sadjadi S.; Akbari M.; Kahangi F. G.; Heravi M. M. Polyhedron 2020, 179, 114375. |
| [14] | Tian H.; Liu S.; Zhang Z.; Dang T.; Lu Y.; Liu S. ACS Sustainable Chem. Eng. 2021, 9, 4660. |
| [15] | Zabihzadeh M.; Omidi A.; Shirini F.; Tajik H.; Langarudi M. S. N. J. Mol. Struct. 2020, 1206, 127730. |
| [16] | Wen H.; Xie J.; Zhou Y.; Zhou Y.; Wang J. Catal. Sci. Technol. 2019, 9, 5725. |
| [17] | Xu Q.; Niu Y.; Wang G.; Li Y.; Zhao Y.; Singh V.; Niu J.; Wang J. Mol. Catal. 2018, 453, 93. |
| [18] | Taher A.; Lee D.; Lee B.; Lee I. Synlett. 2016, 27, 1433. |
| [19] | Modak A.; Mondal J.; Bhaumik A. Appl. Catal. A: Gen. 2013, 459, 41. |
| [20] | Rong N.; Qiu T.; Qian R.; Lu L.; Huang X.; Ma Z.; Cui C. Inorg. Chem. Commun. 2017, 86, 98. |
| [21] | Blanco-Ania D.; Valderas-Cortina C.; Hermkens P. H. H.; Sliedregt L. A. J. M.; Scheeren H. W.; Rutjes F. P. J. T. Molecules 2010, 15, 2269. |
| [22] | Balalaie S.; Bararjanian M.; Hekmat S.; Salehi P. Synth. Commun. 2006, 36, 3703. |
| [23] | Xu H.; Pan L.; Fang X.; Liu B.; Zhang W.; Lu M.; Chang H. Tetrahedron Lett. 2017, 58, 2360. |
| [24] | Li J.; Lear M. J.; Hayashi Y. Chem.-Eur. J. 2021, 27, 5901. |
| [25] | Shi D. Q.; Chen J.; Zhuang Q. Y.; Wang X. S.; Hu H. W. Chin. Chem. Lett. 2003, 14, 1242. |
| [26] | Tukhtaev H. B.; Ivanov K. L.; Bezzubov S. I.; Cheshkov D. A.; Melnikov M. Y.; Budynina E. M. Org. Lett. 2019, 21, 1087. |
| [27] | Ren Y. M.; Cai C. Catal. Lett. 2007, 118, 134. |
| [28] | Wiles C.; Watts P. Haswell S. J. Lab Chip 2007, 7, 322. |
| [29] | Yadav J. S.; Reddy B. V. S.; Basak A. K.; Visali B.; Narsaiah A. V.; Nagaiah K. Eur. J. Org. Chem. 2004, 2004, 546. |
/
| 〈 |
|
〉 |