

Chinese Journal of Organic Chemistry >
Basicity-Tuned Selectivity: Synthesis of Benzimidazolone and Benzodiazepine from N-Alkyl-N-(2-(pyridin-2-ylamino)-phenyl)formamides
Received date: 2021-07-29
Revised date: 2021-09-29
Online published: 2021-12-31
Supported by
Xinjiang Bingtuan Young and Middle-Aged Leading Scientists Program(2020CB027); Shihezi Young and Middle-Aged Leading Scientists Program(2019RC01); Open Sharing Fund for the Large-scale Instruments and Equipment of Shihezi University.
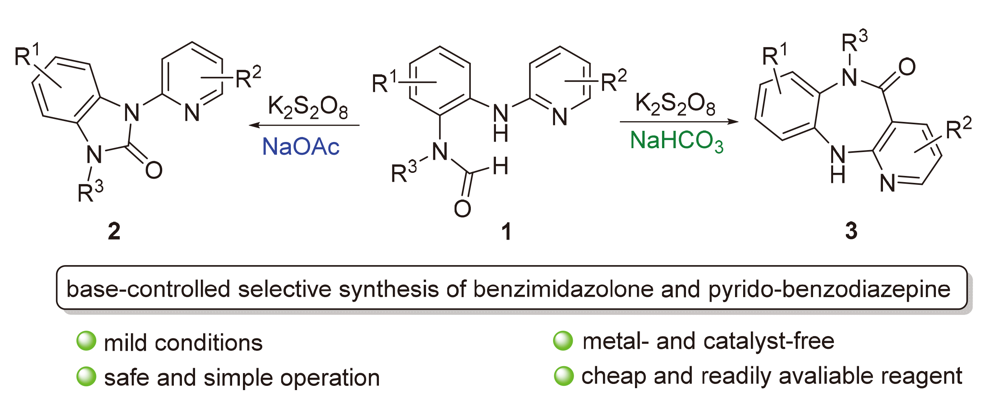
A base-controlled strategy for the selective preparation of benzimidazolone and pyrido-benzodiazepine derivatives was developed. The N-alkyl-N-(2-(pyridin-2-ylamino)phenyl)formamides underwent selective coupling to synthesize a series of benzimidazolones when NaOAc was used as bases and employed K2S2O8 as oxidants. By changing bases to NaHCO3, a series of benzodiazepines was obtained. The possible reaction mechanism was proposed based on the radical-trapping experiments. The applicability of this protocol is demonstrated by scale-up experiments and the functionalization of benzodiazepine products.
Key words: benzimidazolone; benzodiazepine; oxidant-induced; metal-free
Yubin Wang , Cheng Guo , Sheng Tao , Jichang Liu , Jigang Zhao , Ning Liu , Bin Dai . Basicity-Tuned Selectivity: Synthesis of Benzimidazolone and Benzodiazepine from N-Alkyl-N-(2-(pyridin-2-ylamino)-phenyl)formamides[J]. Chinese Journal of Organic Chemistry, 2022 , 42(4) : 1146 -1162 . DOI: 10.6023/cjoc202107062
| [1] | (a) Poupaert, J.; Carato, P.; Colacino, E. Curr. Med. Chem. 2005, 12, 877. |
| [1] | (b) Welsch, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347. |
| [1] | (c) Lo, H. Y.; Nemoto, P. A.; Kim, J. M.; Hao, M.-H.; Qian, K. C.; Farrow, N. A.; Albaugh, D. R.; Fowler, D. M.; Schneiderman, R. D.; Michael August, E.; Martin, L.; Hill-Drzewi, M.; Pullen, S. S.; Takahashi, H.; De Lombaert, S. Bioorg. Med. Chem. Lett. 2011, 21, 4533. |
| [1] | (d) Filippakopoulos, P.; Qi, J.; Picaud, S.; Shen, Y.; Smith, W. B.; Fedorov, O.; Morse, E. M.; Keates, T.; Hickman, T. T.; Felletar, I.; Philpott, M.; Munro, S.; Mckeown, M. R.; Wang, Y.; Christie, A. L.; West, N.; Cameron, M. J.; Schwartz, B.; Heightman, T. D.; La Thangue, N.; French, C. A.; Wiest, O.; Kung, A. L.; Knapp, S.; Bradner, J. E. Nature 2010, 468, 1067. |
| [1] | (e) Králová, P.; Maloň, M.; Soural, M. ACS Comb. Sci. 2017, 19, 770. |
| [1] | (f) Kundu, P.; Mondal, A.; Das, B.; Chowdhury, C. Adv. Synth. Catal. 2015, 357, 3737. |
| [1] | (g) Carlier, P. R.; Zhao, H. W.; Macquarrie-Hunter, S. L.; Deguzman, J. C.; Hsu, D. C. J. Am. Chem. Soc. 2006, 128, 15215. |
| [1] | (h) Al-Tel, T. H.; Al-Qawasmeh, R. A.; Schmidt, M. F.; Al-Aboudi, A.; Rao, S. N.; Sabri, S. S.; Voelter, W. J. Med. Chem. 2009, 52, 6484. |
| [1] | (i) Wang, Y. Y.; Ling, B. P.; Liu, P.; Bi, S. W. Organometallics 2018, 37, 3035. |
| [2] | Meanwell, N. A.; Sit, S.-Y.; Gao, J.; Boissard, C. G.; Lum-Ragan, J.; Dworetzky, S. I.; Gribkoff, V. K. Bioorg. Med. Chem. Lett. 1996, 6, 1641. |
| [3] | (a) P. Barot,, K.; Nikolova, S.; Ivanov, I.; D. Ghate,, M. Mini-Rev. Med. Chem. 2013, 13, 1421. |
| [3] | (b) Yu, K.-L.; Sin, N.; Civiello, R. L.; Wang, X. A.; Combrink, K. D.; Gulgeze, H. B.; Venables, B. L.; Wright, J. J. K.; Dalterio, R. A.; Zadjura, L.; Marino, A.; Dando, S.; D’arienzo, C.; Kadow, K. F.; Cianci, C. W.; Li, Z.; Clarke, J.; Genovesi, E. V.; Medina, I.; Lamb, L.; Colonno, R. J.; Yang, Z.; Krystal, M.; Meanwell, N. A. Bioorg. Med. Chem. Lett. 2007, 17, 895. |
| [3] | (c) Yu, K.-L.; Wang, X. A.; Civiello, R. L.; Trehan, A. K.; Pearce, B. C.; Yin, Z. W.; Combrink, K. D.; Gulgeze, H. B.; Zhang, Y.; Kadow, K. F.; Cianci, C. W.; Clarke, J.; Genovesi, E. V.; Medina, I.; Lamb, L.; Wyde, P. R.; Krystal, M.; Meanwell, N. A. Bioorg. Med. Chem. Lett. 2006, 16, 1115. |
| [4] | (a) Monforte, A.-M.; Logoteta, P.; Ferro, S.; Luca, L. D.; Iraci, N.; Maga, G.; Clercq, E. D.; Pannecouque, C.; Chimirri, A. Biorg. Med. Chem. 2009, 17, 5962. |
| [4] | (b) Monforte, A.-M.; Logoteta, P.; Luca, L. D.; Iraci, N.; Ferro, S.; Maga, G.; De Clercq, E.; Pannecouque, C.; Chimirri, A. Biorg. Med. Chem. 2010, 18, 1702. |
| [5] | Gustin, D. J.; Sehon, C. A.; Wei, J. M.; Cai, H.; Meduna, S. P.; Khatuya, H.; Sun, S. Q.; Gu, Y.; Jiang, W.; Thurmond, R. L.; Karlsson, L.; Edwards, J. P. Bioorg. Med. Chem. Lett. 2005, 15, 1687. |
| [6] | Kawamoto, H.; Nakashima, H.; Kato, T.; Arai, S.; Kamata, K.; Iwasawa, Y. Tetrahedron 2001, 57, 981. |
| [7] | Diao, X. Q.; Wang, Y. J.; Jiang, Y. W.; Ma, D. W. J. Org. Chem. 2009, 74, 7974. |
| [8] | Mclaughlin, M.; Palucki, M.; Davies, I. W. Org. Lett. 2006, 8, 3311. |
| [9] | Beyer, A.; Reucher, C. M. M.; Bolm, C. Org. Lett. 2011, 13, 2876. |
| [10] | (a) Meng, Y. G.; Wang, B. N.; Ren, L. N.; Zhao, Q. L.; Yu, W. Q.; Chang, J. B. New J. Chem. 2018, 42, 13790. |
| [10] | (b) Yu, J. P.; Gao, C.; Song, Z. X.; Yang, H. J.; Fu, H. Eur. J. Org. Chem. 2015, 2015, 5869. |
| [11] | Youn, S. W.; Kim, Y. H. Org. Lett. 2016, 18, 6140. |
| [12] | Xu, F.; Long, H.; Song, J. S.; Xu, H.-C. Angew. Chem., Int. Ed. 2019, 58, 9017. |
| [13] | Li, J.-S.; Yang, P.-P.; Xie, X.-Y.; Jiang, S.; Tao, L.; Li, Z.-W.; Lu, C.-H.; Liu, W.-D. Adv. Synth. Catal. 2020, 362, 1977. |
| [14] | (a) Lima, H. M.; Lovely, C. J. Org. Lett. 2011, 13, 5736. |
| [14] | (b) Hamm, M. L.; Billig, K. Org. Biomol. Chem. 2006, 4, 4068. |
| [14] | (c) Bon, R. S.; Sprenkels, N. E.; Koningstein, M. M.; Schmitz, R. F.; De Kanter, F. J. J.; Dömling, A.; Groen, M. B.; Orru, R. V. A. Org. Biomol. Chem. 2008, 6, 130. |
| [15] | Li, D. Z.; Ollevier, T. Org. Lett. 2019, 21, 3572. |
| [16] | Chernyshev, V. M.; Khazipov, O. V.; Shevchenko, M. A.; Chernenko, A. Y.; Astakhov, A. V.; Eremin, D. B.; Pasyukov, D. V.; Kashin, A. S.; Ananikov, V. P. Chem. Sci. 2018, 9, 5564. |
| [17] | Ruiz, J.; Mesa, A. F. Chem.-Eur. J. 2014, 20, 102. |
| [18] | Fader, L. D.; Bethell, R.; Bonneau, P.; Bös, M.; Bousquet, Y.; Cordingley, M. G.; Coulombe, R.; Deroy, P.; Faucher, A.-M.; Gagnon, A.; Goudreau, N.; Grand-Maître, C.; Guse, I.; Hucke, O.; Kawai, S. H.; Lacoste, J.-E.; Landry, S.; Lemke, C. T.; Malenfant, E.; Mason, S.; Morin, S.; O’meara, J.; Simoneau, B.; Titolo, S.; Yoakim, C. Bioorg. Med. Chem. Lett. 2011, 21, 398. |
| [19] | Eltze, M.; Mutschler, E.; Lambrecht, G. Eur. J. Pharmacol. 1992, 211, 283. |
| [20] | Hargrave, K. D.; Proudfoot, J. R.; Grozinger, K. G.; Cullen, E.; Kapadia, S. R.; Patel, U. R.; Fuchs, V. U.; Mauldin, S. C.; Vitous, J.; Behnke, M. L.; Klunder, J. M.; Pal, K.; Skiles, J. W.; Mcneil, D. W.; Rose, J. M.; Chow, G. C.; Skoog, M. T.; Wu, J. C.; Schmidt, G.; Engel, W. W.; Eberlein, W. G.; Saboe, T. D.; Campbell, S. J.; Rosenthal, A. S.; Adams, J. J. Med. Chem. 1991, 34, 2231. |
| [21] | Pèpe, G.; Reboul, J.-P.; Oddon, Y. Eur. J. Med. Chem. 1989, 24, 1. |
| [22] | (a) Yuan, S.; Yue, Y.-L.; Zhang, D.-Q.; Zhang, J.-Y.; Yu, B.; Liu, H.-M. Chem. Commun. 2020, 56, 11461. |
| [22] | (b) Hwang, J.; Borgelt, L.; Wu, P. ACS Comb. Sci. 2020, 22, 495. |
| [22] | (c) Velasco-Rubio, Á.; Varela, J. A.; Saá, C. Adv. Synth. Catal. 2020, 362, 4861. |
| [22] | (d) Dagar, A.; Kim, I. Org. Biomol. Chem. 2020, 18, 9836. |
| [22] | (e) Archer, G. A.; Sternbach, L. H. Chem. Rev. 1968, 68, 747. |
| [23] | (a) Chobanian, H. R.; Guo, Y.; Liu, P.; Lanza, T. J.; Chioda, M.; Chang, L.; Kelly, T. M.; Kan, Y. Q.; Palyha, O.; Guan, X.-M.; Marsh, D. J.; Metzger, J. M.; Raustad, K.; Wang, S.-P.; Strack, A. M.; Gorski, J. N.; Miller, R.; Pang, J. M.; Lyons, K.; Dragovic, J.; Ning, J. G.; Schafer, W. A.; Welch, C. J.; Gong, X. Y.; Gao, Y.-D.; Hornak, V.; Reitman, M. L.; Nargund, R. P.; Lin, L. S. Biorg. Med. Chem. 2012, 20, 2845. |
| [23] | (b) Liu, P.; Lanza, T. J.; Chioda, M.; Jones, C.; Chobanian, H. R.; Guo, Y.; Chang, L.; Kelly, T. M.; Kan, Y. Q.; Palyha, O.; Guan, X.-M.; Marsh, D. J.; Metzger, J. M.; Ramsay, K.; Wang, S.-P.; Strack, A. M.; Miller, R.; Pang, J. M.; Lyons, K.; Dragovic, J.; Ning, J. G.; Schafer, W. A.; Welch, C. J.; Gong, X. Y.; Gao, Y.-D.; Hornak, V.; Ball, R. G.; Tsou, N.; Reitman, M. L.; Wyvratt, M. J.; Nargund, R. P.; Lin, L. S. ACS Med. Chem. Lett. 2011, 2, 933. |
| [23] | (c) Matsufuji, T.; Shimada, K.; Kobayashi, S.; Ichikawa, M.; Kawamura, A.; Fujimoto, T.; Arita, T.; Hara, T.; Konishi, M.; Abe-Ohya, R.; Izumi, M.; Sogawa, Y.; Nagai, Y.; Yoshida, K.; Abe, Y.; Kimura, T.; Takahashi, H. Biorg. Med. Chem. 2015, 23, 89. |
| [23] | (d) Failli, A. A.; Shumsky, J. S.; Steffan, R. J.; Caggiano, T. J.; Williams, D. K.; Trybulski, E. J.; Ning, X.; Lock, Y.; Tanikella, T.; Hartmann, D.; Chan, P. S.; Park, C. H. Bioorg. Med. Chem. Lett. 2006, 16, 954. |
| [24] | Maddess, M. L.; Li, C. M. Organometallics 2019, 38, 81. |
| [25] | Novelli, F.; Sparatore, A.; Tasso, B.; Sparatore, F. Bioorg. Med. Chem. Lett. 1999, 9, 3031. |
| [26] | Shi, F. Q.; Xu, X. X.; Zheng, L. Y.; Dang, Q.; Bai, X. J. Comb. Chem. 2008, 10, 158. |
| [27] | (a) Zhu, H. Q.; Shang, T. B.; Lu, Z. H.; Luo, F.; Zhu, G. G. Chin. J. Org. Chem. 2020, 40, 3410. (in Chinese) |
| [27] | ( 朱海倩, 商甜波, 卢增辉, 罗芳, 朱钢国, 有机化学, 2020, 40, 3410.) |
| [27] | (b) Tian, S. H.; Luo, T.; Zhu, Y. P.; Wan, J.-P. Chin. Chem. Lett. 2020, 31, 3073. |
| [27] | (c) Fu, L. Q.; Liu, Y. Y.; Wan, J.-P. Org. Lett. 2021, 23, 4363. |
| [27] | (d) Yu, Q.; Liu, Y. Y.; Wan, J.-P. Org. Chem. Front. 2020, 7, 2770. |
| [27] | (e) Chen, Q. W.; Yang, Y. C.; Wang, X.; Zhang, Q.; Li, D. Chin. J. Org. Chem. 2020, 40, 451. (in Chinese) |
| [27] | ( 陈倩雯, 杨耀成, 王霞, 张谦, 李栋, 有机化学, 2020, 40, 451.) |
| [27] | (f) Zhao, B. L.; Liu, Y. Y. Synthesis 2020, 52, 3211. |
| [28] | Tao, S.; Bu, Q. Q.; Shi, Q. Q.; Wei, D. H.; Dai, B.; Liu, N. Chem. Eur. J. 2020, 26, 3252. |
| [29] | (a) Karthikeyan, J.; Cheng, C.-H. Angew. Chem., Int. Ed. 2011, 50, 9880. |
| [29] | (b) Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147. |
| [29] | (c) Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094. |
| [29] | (d) Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792. |
| [29] | (e) Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215. |
| [29] | (f) Liu, C.; Zhang, H.; Shi, W.; Lei, A. W. Chem. Rev. 2011, 111, 1780. |
| [30] | (a) Liu, Q.; Xie, G. Q.; Wang, Q.; Mo, Z. D.; Li, C.; Ding, S. J.; Wang, X. X. Tetrahedron 2019, 75, 130490. |
| [30] | (b) Minisci, F.; Citterio, A.; Giordano, C. Acc. Chem. Res. 1983, 16, 27. |
| [30] | (c) Yan, K.; Yang, D. S.; Wei, W.; Wang, F.; Shuai, Y. Y.; Li, Q. N.; Wang, H. J. Org. Chem. 2015, 80, 1550. |
| [30] | (d) Mandal, S.; Bera, T.; Dubey, G.; Saha, J.; Laha, J. K. ACS Catal. 2018, 8, 5085. |
| [30] | (e) Zhang, Z.; Jia, C.; Kong, X.; Hussain, M.; Liu, Z.; Liang, W.; Jiang, L.; Jiang, H.; Ma, J. ACS Sustainable Chem. Eng. 2020, 8, 16463. |
| [30] | (f) Zhu, Y. C.; Huang, K. M.; Pan, J.; Qiu, X.; Luo, X.; Qin, Q. X.; Wei, J. L.; Wen, X. J.; Zhang, L. Z.; Jiao, N. Nat. Commun. 2018, 9, 2625. |
| [31] | (a) Shi, Z. Z.; Glorius, F. Chem. Sci. 2013, 4, 829. |
| [31] | (b) Wang, X. Y.; Wang, S. C.; Gao, Y. M.; Sun, H.; Liang, X. A.; Bu, F. X.; Abdelilah, T.; Lei, A. W. Org. Lett. 2020, 22, 5429. |
| [31] | (c) Chen, Z. C.; Zhang, H.; Zhou, S. F.; Cui, X. L. Chin. J. Org. Chem. 2020, 40, 3866. (in Chinese) |
| [31] | ( 陈志超, 张红, 周树锋, 崔秀灵, 有机化学, 2020, 40, 3866.) |
| [32] | (a) Yi, H.; Zhang, G. T.; Wang, H. M.; Huang, Z. Y.; Wang, J.; Singh, A. K.; Lei, A. W. Chem. Rev. 2017, 117, 9016. |
| [32] | (b) Pan, G.-A.; Li, Y.; Li, J.-H. Org. Chem. Front. 2020, 7, 2486. |
| [33] | (a) Hollóczki, O.; Terleczky, P.; Szieberth, D.; Mourgas, G.; Gudat, D.; Nyulászi, L. J. Am. Chem. Soc. 2011, 133, 780. |
| [33] | (b) Tao, S.; Guo, C.; Liu, N.; Dai, B. Organometallics 2017, 36, 4432. |
| [34] | (a) Khan, D.; Mukhtar, S.; Alsharif, M. A.; Alahmdi, M. I.; Ahmed, N. Tetrahedron Lett. 2017, 58, 3183. |
| [34] | (b) Prasad, V.; Kale, R. R.; Mishra, B. B.; Kumar, D.; Tiwari, V. K. Org. Lett. 2012, 14, 2936. |
| [35] | Chen, F.; Chen, D. T.; Shi, L.; Liu, N.; Dai, B. J. CO2 Util. 2016, 16, 391. |
| [36] | Wang, Y.-B.; Liu, B.-Y.; Bu, Q. Q.; Dai, B.; Liu, N. Adv. Synth. Catal. 2020, 362, 2930. |
/
| 〈 |
|
〉 |