

Chinese Journal of Organic Chemistry >
Synthesis of Nitrile via Electrochemical Appel Reaction
Received date: 2022-02-06
Revised date: 2022-03-11
Online published: 2022-03-22
Supported by
National Natural Science Foundation of China(22001217); Science and Technology Program of Sichuan Province(2021ZYD0064); Meritocracy Research Funds of China West Normal University(17YC020); Fundamental Research Funds of China West Normal University(19D037); Fundamental Research Funds of China West Normal University(19E034)
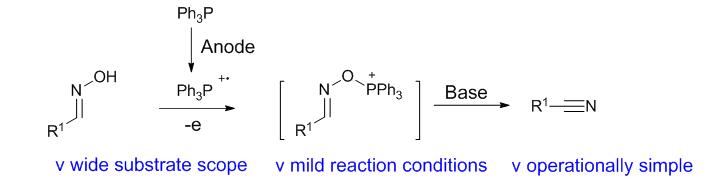
An electrochemical Appel reaction was developed for the synthesis of nitriles. This protocol features operationally simplicity, mild reaction conditions and environmental friendliness, enabling synthesis of various aromatic and aliphatic nitriles. Based on the controlled experiments and cyclic voltammetry (CV) experimental results, an electrochemical Appel reaction mechanism was proposed to explain the reaction process.
Haiqiong Li , Mengyun Yin , Fenfen Xie , Zhengbing Zhang , Pan Han , Linhai Jing . Synthesis of Nitrile via Electrochemical Appel Reaction[J]. Chinese Journal of Organic Chemistry, 2022 , 42(7) : 2229 -2235 . DOI: 10.6023/cjoc202202007
| [1] | (a) Dworczak, R.; Fabian, W. M. F.; Biza, P.; Weikmann, M.; Junek, H. Dyes Pigm. 1995, 28, 297. |
| [1] | (b) Dworczak, R.; Fabian, W. M. F.; Pawar, B. N.; Junek, H. Dyes Pigm. 1995, 29, 65. |
| [1] | (c) Pearce, E. M.; Weil, E. D.; Barinov, V. Y. Fire Smart Polymers (Fire and Polymers), American Chemical Society, 2001, pp. 37-48. |
| [1] | (d) Amr, M. A.; Mohamed, H. E.; Mohamed, S. E.; Hesham, R. E.-S.; Ismail, A. A. Curr. Org. Synth. 2018, 15, 487. |
| [2] | Fatiadi, A. J. In Preparation and Synthetic Applications of Cyano Compounds, Eds.: Patai, S.; Rappaport, Z., Wiley, New York, 1983. |
| [3] | (a) Iyengar, B. S.; Dorr, R. T.; Remers, W. A. J. Med. Chem. 2004, 47, 218. |
| [3] | (b) Romero, M.; Renard, P.; Caignard, D.-H.; Atassi, G.; Solans, X.; Constans, P.; Bailly, C.; Pujol, M. D. J. Med. Chem. 2007, 50, 294. |
| [4] | Pascual, E.; Sivera, F.; Yasothan, U.; Kirkpatrick, P. Nat. Rev. Drug Discov. 2009, 8, 191. |
| [5] | Patat, A.; Paty, I.; Hindmarch, I. Hum. Psychopharmacol. 2001, 16, 369. |
| [6] | (a) Sica, D. A.; Prisant, L. M. J. Clin. Hypertens. (Shelton, CT, U. S.) 2007, 9, 1. |
| [6] | (b) Cooper-DeHoff, R. M.; Handberg, E. M.; Mancia, G.; Zhou, Q.; Champion, A.; Legler, U. F.; Pepine, C. J. Expert Rev. Cardiovasc. Ther. 2009, 7, 1329. |
| [7] | Noble, S.; McTavish, D. Drugs 1995, 50, 1032. |
| [8] | (a) Shu, Z.; Ye, Y.; Deng, Y.; Zhang, Y.; Wang, J. Angew. Chem., Int. Ed. 2013, 52, 10573. |
| [8] | (b) Liu, J.; Zheng, H.-X.; Yao, C.-Z.; Sun, B.-F.; Kang, Y.-B. J. Am. Chem. Soc. 2016, 138, 3294. |
| [8] | (c) Ge, J.-J.; Yao, C.-Z.; Wang, M.-M.; Zheng, H.-X.; Kang, Y.-B.; Li, Y. Org. Lett. 2016, 18, 228. |
| [8] | (d) Yu, L.; Li, H.; Zhang, X.; Ye, J.; Liu, J.; Xu, Q.; Lautens, M. Org. Lett. 2014, 16, 1346. |
| [8] | (e) Zhuang, Y.-J.; Liu, J.; Kang, Y.-B. Tetrahedron Lett. 2016, 57, 5700. |
| [9] | (a) Zhou, S.; Addis, D.; Das, S.; Junge, K.; Beller, M. Chem. Commun. 2009, 4883. |
| [9] | (b) Shipilovskikh, S. A.; Vaganov, V. Y.; Denisova, E. I.; Rubtsov, A. E.; Malkov, A. V. Org. Lett. 2018, 20, 728. |
| [10] | (a) Zhou, W.; Zhang, L.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7094. |
| [10] | (b) Tseng, K.-N. T.; Rizzi, A. M.; Szymczak, N. K. J. Am. Chem. Soc. 2013, 135, 16352. |
| [10] | (c) Guo, S.; Wan, G.; Sun, S.; Jiang, Y.; Yu, J.-T.; Cheng, J. Chem. Commun. 2015, 51, 5085. |
| [11] | (a) Anderson, B. A.; Bell, E. C.; Ginah, F. O.; Harn, N. K.; Pagh, L. M.; Wepsiec, J. P. J. Org. Chem. 1998, 63, 8224. |
| [11] | (b) Zanon, J.; Klapars, A.; Buchwald, S. L. J. Am. Chem. Soc. 2003, 125, 2890. |
| [11] | (c) Cristau, H.-J.; Ouali, A.; Spindler, J.-F.; Taillefer, M. Chem.-Eur. J. 2005, 11, 2483. |
| [11] | (d) Pan, S.; Wu, F.; Yu, R.; Chen, W. J. Org. Chem. 2016, 81, 1558. |
| [11] | (e) Yan, G.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2017, 359, 4068. |
| [12] | (a) Fang, C.; Li, M.; Hu, X.; Mo, W.; Hu, B.; Sun, N.; Jin, L.; Shen, Z. RSC Adv. 2017, 7, 1484. |
| [12] | (b) Murugesan, K.; Senthamarai, T.; Sohail, M.; Sharif, M.; Kalevaru, N. V.; Jagadeesh, R. V. Green Chem. 2018, 20, 266. |
| [12] | (c) Chen, H.; Sun, S.; Xi, H.; Hu, K.; Zhang, N.; Qu, J.; Zhou, Y. Tetrahedron Lett. 2019, 60, 1434. |
| [12] | (d) Zhan, W.; Tong, M.; Ji, L.; Zhang, H.; Ge, Z.; Wang, X.; Li, R.. Chin. Chem. Lett. 2019, 30, 973. |
| [12] | (e) Mudshinge, S. R.; Potnis, C. S.; Xu, B.; Hammond, G. B. Green Chem. 2020, 22, 4161. |
| [12] | (f) Hua, M.; Song, J.; Huang, X.; Liu, H.; Fan, H.; Wang, W.; He, Z.; Liu, Z.; Han, B. Angew. Chem., Int. Ed. 2021, 60, 21479. |
| [13] | (a) Xu, J.-H.; Jiang, Q.; Guo, C.-C. J. Org. Chem. 2013, 78, 11881. |
| [13] | (b) Preger, Y.; Root, T. W.; Stahl, S. S. ACS Omega 2018, 3, 6091. |
| [13] | (c) Vanoye, L.; Hammoud, A.; Gérard, H.; Barnes, A.; Philippe, R.; Fongarland, P.; de Bellefon, C.; Favre-Réguillon, A. ACS Catal. 2019, 9, 9705. |
| [13] | (d) Murata, Y.; Iwasa, H.; Matsumura, M.; Yasuike, S. Chem. Pharm. Bull. 2020, 68, 679. |
| [13] | (e) Takahashi, Y.; Tsuji, H.; Kawatsura, M. J. Org. Chem. 2020, 85, 2654. |
| [13] | (f) Lu, D.; Cui, J.; Yang, S.; Gong, Y. ACS Catal. 2021, 11, 4288. |
| [13] | (g) Xiao, J.; Guo, F.; Li, Y.; Li, F.; Li, Q.; Tang, Z.-L. J. Org. Chem. 2021, 86, 2028. |
| [14] | (a) Li, Y.-T.; Liao, B.-S.; Chen, H.-P.; Liu, S.-T. Synthesis 2011, 2639. |
| [14] | (b) Ma, X.-Y.; He, Y.; Lu, T.-T.; Lu, M. Tetrahedron 2013, 69, 2560. |
| [14] | (c) Ghosh, P.; Pariyar, G. C.; Saha, B.; Subba, R. Synth. Commun. 2016, 46, 685. |
| [14] | (d) Hyodo, K.; Kitagawa, S.; Yamazaki, M.; Uchida, K. Chem. Asian J. 2016, 11, 1348. |
| [14] | (e) Rapeyko, A.; Climent, M. J.; Corma, A.; Concepción, P.; Iborra, S. ACS Catal. 2016, 6, 4564. |
| [14] | (f) Sun, D.; Kitamura, E.; Yamada, Y.; Sato, S. Green Chem. 2016, 18, 3389. |
| [14] | (g) Ding, R.; Liu, Y.; Han, M.; Jiao, W.; Li, J.; Tian, H.; Sun, B. J. Org. Chem. 2018, 83, 12939. |
| [14] | (h) Zhang, D.; Huang, Y.; Zhang, E.; Yi, R.; Chen, C.; Yu, L.; Xu, Q. Adv. Synth. Catal. 2018, 360, 784. |
| [14] | (i) Ma, X.; Liu, D.; Chen, Z. Synth. Commun. 2021, 51, 3261. |
| [15] | (a) Sperry, J. B.; Wright, D. L. Chem. Soc. Rev. 2006, 35, 605. |
| [15] | (b) Jutand, A. Chem. Rev. 2008, 108, 2300. |
| [15] | (c) Yoshida, J.-I.; Kataoka, K.; Horcajada, R.; Nagaki, A. Chem. Rev. 2008, 108, 2265. |
| [15] | (d) Francke, R.; Little, R. D. Chem. Soc. Rev. 2014, 43, 2492. |
| [15] | (e) Yan, M.; Kawamata, Y.; Baran, P. S. Chem. Rev. 2017, 117, 13230. |
| [15] | (f) Ma, C.; Fang, P.; Mei, T.-S. ACS Catal. 2018, 8, 7179. |
| [15] | (g) Moeller, K. D. Chem. Rev. 2018, 118, 4817. |
| [15] | (h) Yoshida, J.-I.; Shimizu, A.; Hayashi, R. Chem. Rev. 2018, 118, 4702. |
| [15] | (i) Marken, F.; Wadhawan, J. D. Acc. Chem. Res. 2019, 52, 3325. |
| [15] | (j) Xiong, P.; Xu, H.-C. Acc. Chem. Res. 2019, 52, 3339. |
| [15] | (k) Yuan, Y.; Lei, A. Acc. Chem. Res. 2019, 52, 3309. |
| [15] | (l) Jiao, K.-J.; Xing, Y.-K.; Yang, Q.-L.; Qiu, H.; Mei, T.-S. Acc. Chem. Res. 2020, 53, 300. |
| [15] | (m) Siu, J. C.; Fu, N.; Lin, S. Acc. Chem. Res. 2020, 53, 547. |
| [16] | (a) Mo, Z.-Y.; Swaroop, T. R.; Tong, W.; Zhang, Y.-Z.; Tang, H.-T.; Pan, Y.-M.; Sun, H.-B.; Chen, Z.-F. Green Chem. 2018, 20, 4428. |
| [16] | (b) Zhang, Y.-Z.; Mo, Z.-Y.; Wang, H.-S.; Wen, X.-A.; Tang, H.-T.; Pan, Y.-M. Green Chem. 2019, 21, 3807. |
| [16] | (c) Wang, X.-Y.; Zhong, Y.-F.; Mo, Z.-Y.; Wu, S.-H.; Xu, Y.-L.; Tang, H.-T.; Pan, Y.-M. Adv. Synth. Catal. 2021, 363, 208. |
| [16] | (d) Wu, Y.; Chen, J.-Y.; Liao, H.-R.; Shu, X.-R.; Duan, L.-L.; Yang, X.-F.; He, W.-M. Green Synth. Catal. 2021, 2, 233. |
| [16] | (e) Yang, Z.; Yu, Y.; Lai, L.; Zhou, L.; Ye, K.; Chen, F.-E. Green Synth. Catal. 2021, 2, 19. |
| [16] | (f) Zhang, S.; Ye, X.; Wojtas, L.; Hao, W.; Shi, X. Green Synth. Catal. 2021, 2, 82. |
| [17] | Libendi, S. S.; Demizu, Y.; Onomura, O. Org. Biomol. Chem. 2009, 7, 351. |
| [18] | (a) Cui, T.; Zhan, Y.; Dai, C.; Lin, J.; Liu, P.; Sun, P. J. Org. Chem. 2021, 86, 15897. |
| [18] | (b) Gao, J.; Weng, X.; Ma, C.; Xu, X.; Fang, P.; Mei, T. Chin. J. Org. Chem. 2021, 41, 3223. (in Chinese) |
| [18] | ( 高君青, 翁信军, 马聪, 徐学涛, 方萍, 梅天胜, 有机化学, 2021, 41, 3223.) |
| [19] | Dai, J.-J.; Huang, Y.-B.; Fang, C.; Guo, Q.-X.; Fu, Y. ChemSusChem 2012, 5, 617. |
| [20] | Ye, J.-Q.; Zhang, Z.-L.; Zha, Z.-G.; Wang, Z.-Y. Chin. Chem. Lett. 2014, 25, 1112. |
| [21] | Qu, Q.; Gao, X.; Gao, J.; Yuan, G. Sci. China Chem. 2015, 58, 747. |
| [22] | Shono, T.; Matsumura, Y.; Tsubata, K.; Kamada, T.; Kishi, K.. J. Org. Chem. 1989, 54, 2249. |
| [23] | Hartmer, M. F.; Waldvogel, S. R. Chem. Commun. 2015, 51, 16346. |
| [24] | (a) Ohmori, H.; Nakai, S.; Sekiguchi, M.; Masui, M. Chem. Pharm. Bull. 1980, 28, 910. |
| [24] | (b) Xu, Z.; Zheng, Y.; Wang, Z.; Shao, X.; Tian, L.; Wang, Y. Chem. Commun. 2019, 55, 15089. |
| [25] | (a) de Andrade, V. S. C.; de Mattos, M. C. S. Curr. Org. Synth. 2015, 12, 309. |
| [25] | (b) Li, Z.; Sun, W.; Wang, X.; Li, L.; Zhang, Y.; Li, C. J. Am. Chem. Soc. 2021, 143, 3536. |
| [26] | (a) Ban, Y.-L.; Dai, J.-L.; Jin, X.-L.; Zhang, Q.-B.; Liu, Q. Chem. Commun. 2019, 55, 9701. |
| [26] | (b) Schäfer, R. J. B.; Monaco, M. R.; Li, M.; Tirla, A.; Rivera- Fuentes, P.; Wennemers, H. J. Am. Chem. Soc. 2019, 141, 18644. |
/
| 〈 |
|
〉 |