化学学报 ›› 2021, Vol. 79 ›› Issue (10): 1257-1264.DOI: 10.6023/A21050223 上一篇 下一篇
研究论文
廖妮a,b, 钟霞a, 梁文斌a, 袁若a, 卓颖a,*( )
)
投稿日期:2021-05-20
发布日期:2021-08-17
通讯作者:
卓颖
基金资助:
Ni Liaoa,b, Xia Zhonga, Wen-Bin Lianga, Ruo Yuana, Ying Zhuoa( )
)
Received:2021-05-20
Published:2021-08-17
Contact:
Ying Zhuo
Supported by:文章分享

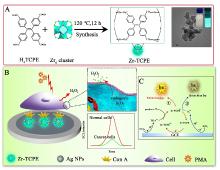
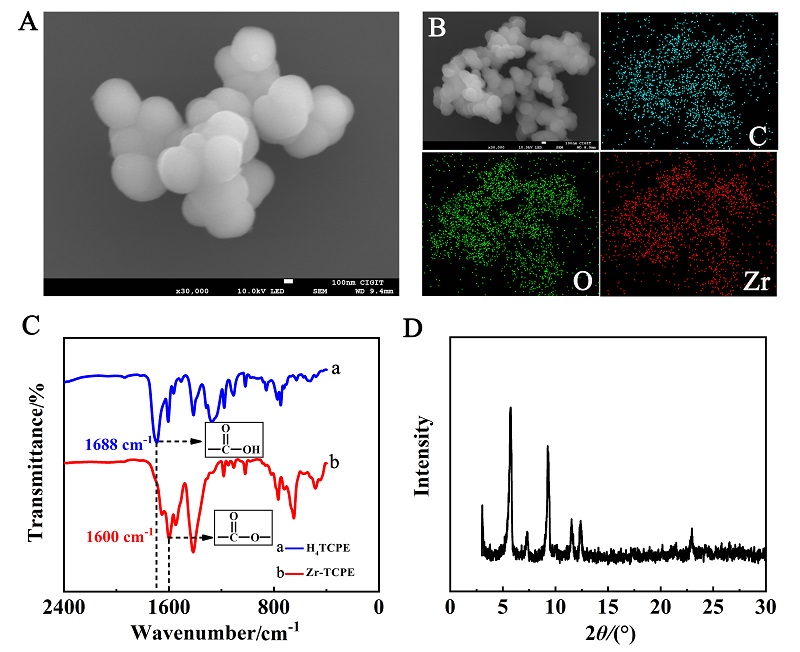
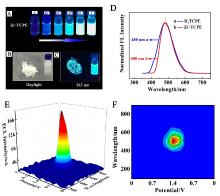
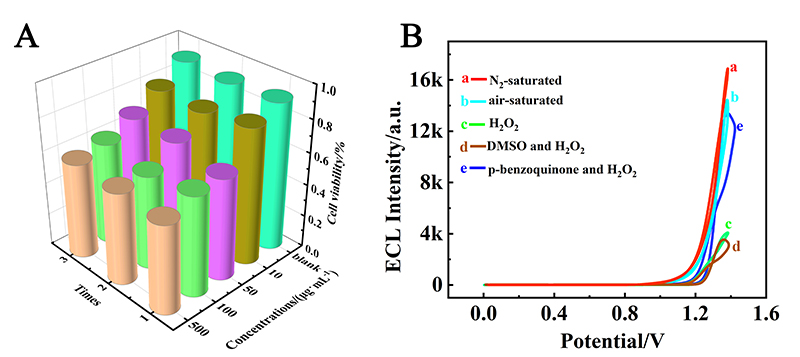
本工作借助金属-有机框架(MOF)结构, 通过配位作用实现对电化学发光(ECL)分子的空间有序组装, 获得了高性能ECL新材料, 发展了孔道限域效应和微纳空间ECL分子有序组装的协同增强ECL新策略. 具体而言, 以非共平面ECL分子1,1,2,2-四(4-羧基苯)乙烯(H4TCPE)为配体采用一步水热法合成了具有优异ECL性能的新型MOF材料(Zr-TCPE). Zr-TCPE通过将发光配体H4TCPE固定在MOF多孔材料上, 一方面其多孔结构可以促进限域空间内物质和电子的传递, 加快电化学反应的效率; 同时, MOF结构的配位键合作用限制了发光基团的自由转动与振动以及分子间的紧密堆积, 从而有效减少了非辐射弛豫和内滤效应. 和H4TCPE的微纳晶体相比, Zr-TCPE的ECL强度提高了3.46倍, ECL效率提高了17.44倍. 采用Zr-TCPE构建了ECL生物传感平台, 成功实现了在药物刺激下肿瘤细胞释放过氧化氢(H2O2)的原位监测, 对临床检测肿瘤细胞具有指导意义.
廖妮, 钟霞, 梁文斌, 袁若, 卓颖. ECL金属-有机框架(MOF)生物传感平台用于肿瘤细胞分泌H2O2的测定[J]. 化学学报, 2021, 79(10): 1257-1264.
Ni Liao, Xia Zhong, Wen-Bin Liang, Ruo Yuan, Ying Zhuo. Metal-organic Frameworks (MOF)-based Novel Electrochemiluminescence Biosensing Platform for Quantification of H2O2 Releasing from Tumor Cells[J]. Acta Chimica Sinica, 2021, 79(10): 1257-1264.

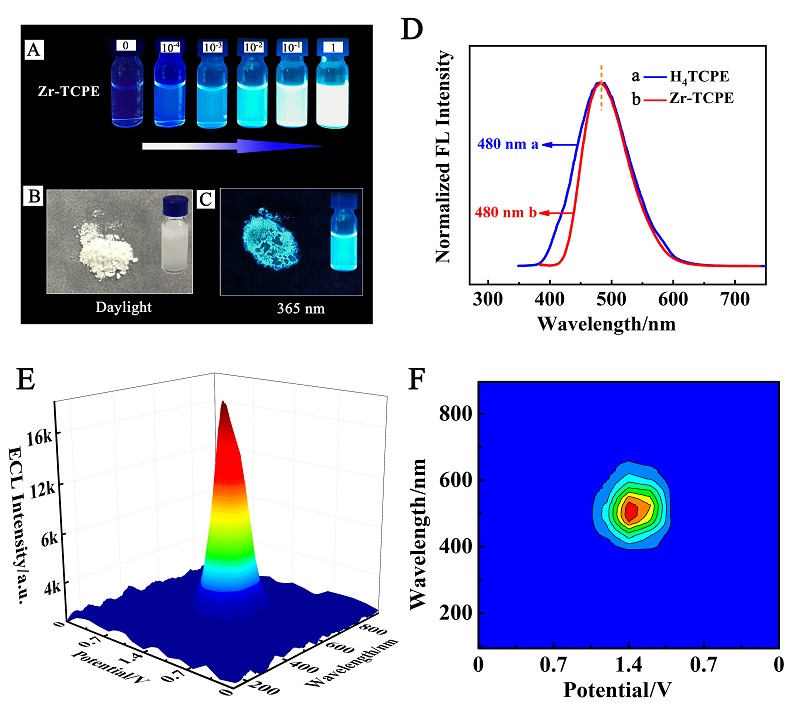
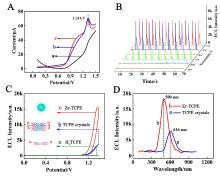

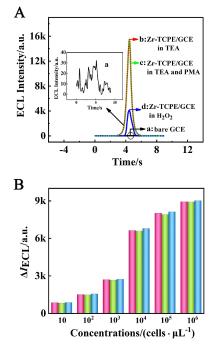
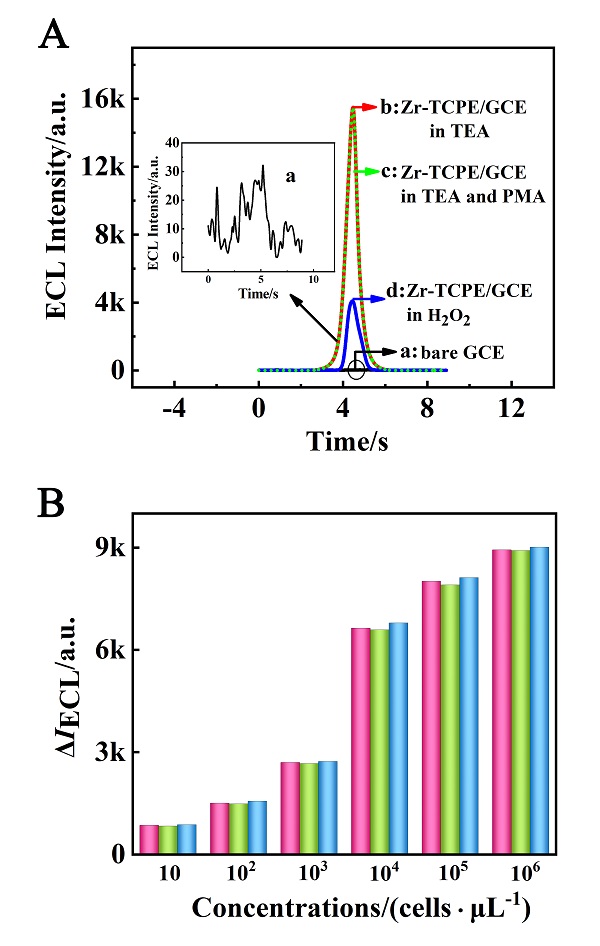

| [1] |
Li, S. J.; Liu, Y.; Ma, Q. Trac-Trends Anal. Chem. 2019, 110, 277.
doi: 10.1016/j.trac.2018.11.019 |
| [2] |
Wang, P.; Shi, Y. H.; Zhang, S. C.; Huang, X. Y.; Zhang, J. J.; Zhang, Y. W.; Si, W. L.; Dong, X. C. Small 2019, 15, 1803791.
doi: 10.1002/smll.201803791 |
| [3] |
Yan, T.; Liu, Z. H.; Song, X. Y.; Zhang, S. S. Acta Chim. Sinica 2020, 78, 657. (in Chinese)
doi: 10.6023/A20040132 |
|
(闫涛, 刘振华, 宋昕玥, 张书圣, 化学学报, 2020, 78, 657.)
doi: 10.6023/A20040132 |
|
| [4] |
Xuan, W. J.; Xia, Y. H.; Li, T.; Wang, L. L.; Liu, Y. L.; Tan, W. H. J. Am. Chem. Soc. 2020, 142, 937.
doi: 10.1021/jacs.9b10755 |
| [5] |
Hao, X. J.; Zhao, S. F.; Zhang, C. M.; Hu, F. X.; Yang, H. B.; Guo, C. X. Mater. Rep. 2021, 35, 3183. (in Chinese)
|
|
郝喜娟, 赵沈飞, 张春媚, 胡芳馨, 杨鸿斌, 郭春显, 材料导报, 2021, 35, 3183).
|
|
| [6] |
Liu, G.; Ma, C.; Jin, B. K.; Chen, Z. X.; Zhu, J. J. Anal. Chem. 2018, 90, 4801.
doi: 10.1021/acs.analchem.8b00194 |
| [7] |
Zhang, L. W.; Chen, Q. X.; Wang, J. Y. Acta Chim. Sinica 2020, 78, 642. (in Chinese)
doi: 10.6023/A20040116 |
|
(张留伟, 陈麒先, 王静云, 化学学报, 2020, 78, 642.)
doi: 10.6023/A20040116 |
|
| [8] |
Jiang, T.; Sun, X. X.; Wei, L. L.; Li, M. G. Anal. Chim. Acta 2020, 1135, 132.
doi: 10.1016/j.aca.2020.09.040 pmid: 33070850 |
| [9] |
Notaro, A.; Gasser, G. Chem. Soc. Rev. 2017, 46, 7317.
doi: 10.1039/c7cs00356k pmid: 29027562 |
| [10] |
Würthner, F.; Saha-Möller, C. R.; Fimmel, B.; Ogi, S.; Leowanawat, P.; Schmidt, D. Chem. Rev. 2016, 116, 962.
doi: 10.1021/acs.chemrev.5b00188 pmid: 26270260 |
| [11] |
Nowak-Król, A.; Shoyama, K.; Stolte, M.; Wurthner, F. Chem. Commun. 2018, 54, 13763.
doi: 10.1039/C8CC07640E |
| [12] |
Zhang, X. J.; Yuan, G. D.; Li, Q. S.; Wang, B.; Zhang, X. H.; Zhang, R. Q.; Chang, J. C.; Lee, C. S.; Lee, S. T. Chem. Mater. 2008, 20, 6945.
doi: 10.1021/cm801896r |
| [13] |
Dick, J. E.; Renault, C.; Kim, B. K.; Bard, A. J. J. Am. Chem. Soc. 2014, 136, 13546.
doi: 10.1021/ja507198r |
| [14] |
Yang, B.; Xiao, J. C.; Wong, J. I.; Guo, J.; Wu, Y. C.; Ong, L. J.; Lao, L. L.; Boey, F.; Zhang, H.; Yang, H. Y.; Zhang, Q. C. J. Phys. Chem. C 2011, 115, 7924.
doi: 10.1021/jp112195k |
| [15] |
Liu, J. L.; Tang, Z. L.; Zhuo, Y.; Chai, Y. Q.; Yuan, R. Anal. Chem. 2017, 89, 9108.
doi: 10.1021/acs.analchem.7b01812 |
| [16] |
Zhao, X. C.; Zhou, W. J.; Lu, C. Anal. Chem. 2017, 89, 10078.
doi: 10.1021/acs.analchem.7b02921 |
| [17] |
Suk, J.; Wu, Z. Y.; Wang, L.; Bard, A. J. J. Am. Chem. Soc. 2011, 133, 14675.
doi: 10.1021/ja203731n |
| [18] |
Lai, R. Y.; Fleming, J. J.; Merner, B. L.; Vermeij, R. J.; Bodwell, G. J.; Bard, A. J. J. Phys. Chem. A 2004, 108, 376.
doi: 10.1021/jp0367981 |
| [19] |
Hu, G. B.; Xiong, C. Y.; Liang, W. B.; Zeng, X. Z.; Xu, H. L.; Yang, Y.; Yao, L. Y.; Yuan, R.; Xiao, D. R. ACS Appl. Mater. Interfaces 2018, 10, 15913.
doi: 10.1021/acsami.8b05038 |
| [20] |
Dong, B. L.; Song, X. Z.; Kong, X. Q.; Wang, C.; Tang, Y. H.; Liu, Y.; Lin, W. Y. Adv. Mater. 2016, 28, 8755.
doi: 10.1002/adma.201602939 |
| [21] |
Wang, F.; Lin, J.; Zhao, T. B.; Hu, D. D.; Wu, T.; Liu, Y. J. Am. Chem. Soc. 2016, 138, 7718.
doi: 10.1021/jacs.6b03662 |
| [22] |
Wang, S. J.; Harris, E.; Shi, J. A.; Chen, A.; Parajuli, S.; Jing, X. H.; Miao, W. Phys. Chem. Chem. Phys. 2010, 12, 10073.
doi: 10.1039/c0cp00545b |
| [23] |
Chen, T. T.; Hu, Y. H.; Cen, Y.; Chu, X.; Lu, Y. J. Am. Chem. Soc. 2013, 135, 11595.
doi: 10.1021/ja4035939 |
| [24] |
Han, Z. L.; Shu, J. N.; Jiang, Q. S.; Cui, H. Anal. Chem. 2018, 90, 6064.
doi: 10.1021/acs.analchem.7b05406 |
| [25] |
Shustova, N. B.; McCarthy, B. D.; Dinca, M. J. Am. Chem. Soc. 2011, 133, 20126.
doi: 10.1021/ja209327q pmid: 22074054 |
| [1] | 王丹, 封波, 张晓昕, 刘亚楠, 裴燕, 乔明华, 宗保宁. 基于热解ZIF-8的氮掺杂碳电化学氧还原合成过氧化氢催化剂[J]. 化学学报, 2022, 80(6): 772-780. |
| [2] | 曾锦跃, 王小双, 张先正, 卓仁禧. 功能化金属-有机框架材料在肿瘤治疗中的研究进展[J]. 化学学报, 2019, 77(11): 1156-1163. |
| [3] | 陈芳芳, 孙晓慧, 姚倩, 李泽荣, 王静波, 李象远. 烷基过氧化氢中氢提取反应类大分子体系的反应能垒与速率常数的精确计算[J]. 化学学报, 2018, 76(4): 311-318. |
| [4] | 何燕萍, 谭衍曦, 张健. 基于尺寸识别和离子交换实现有机染料分离的一例阴离子型MOF[J]. 化学学报, 2014, 72(12): 1228-1232. |
| [5] | 高菊逸, 杜晶辉, 张望, 张宝月, 刘宗彬, 陈艳, 徐小平. 简易型微流控芯片捕获循环肿瘤细胞的研究[J]. 化学学报, 2014, 72(1): 69-74. |
| [6] | 谢文菁, 傅英懿, 马红, 张沫, 范楼珍. 荧光石墨烯量子点制备及其在细胞成像中的应用[J]. 化学学报, 2012, 70(20): 2169-2172. |
| [7] | 唐侃, 陶辉锦, 蒋新宇. 酸性条件下正钛酸钛(IV)中心与过氧化氢反应机理的第一性原理研究[J]. 化学学报, 2012, 70(19): 2091-2096. |
| [8] | 李艳霞, 段晓勇, 李先国, 唐旭利. 水体中壬基酚光降解机理研究[J]. 化学学报, 2012, 70(17): 1819-1826. |
| [9] | 夏前芳, 黄颖娟, 杨雪, 李在均. 新型石墨烯/金/功能导电高分子/过氧化氢生物传感器的制备及应用[J]. 化学学报, 2012, 70(11): 1315-1321. |
| [10] | 杨绍明, 黄爱花, 查文玲, 魏志鹏, 郑龙珍. 基于聚硫堇和层层组装碳纳米管/HRP的过氧化氢传感器研究[J]. 化学学报, 2011, 69(18): 2143-2147. |
| [11] | 李燕, 高艳芳, 刘俞辰, 周宇, 刘进荣. 血红蛋白在二氧化铈修饰电极上的直接电化学[J]. 化学学报, 2010, 68(12): 1161-1166. |
| [12] | 张士国, 卞贺, 夏道宏. CH3SH与H2O2气相反应机理的理论研究[J]. 化学学报, 2010, 68(11): 1050-1056. |
| [13] | 张颖, 王欣, 李来才. 一种非血红素四氮杂轮烯配合物[Fe(III)TMTAA]催化过氧化氢歧化反应机理的理论研究[J]. 化学学报, 2010, 68(07): 633-640. |
| [14] | 曾伟鹏, 李小红, 杜娟, 李建梅, 张平, 胡常伟, 孟祥光. 金属铜配合物催化氧化1-(3,4-二甲氧基苯)乙醇的动力学研究[J]. 化学学报, 2010, 68(01): 27-32. |
| [15] | 华英杰,王崇太,李高仁,童叶翔,李玉光. Keggin型缺位磷钨杂多阴离子的电化学性质及电催化还原过氧化氢[J]. 化学学报, 2009, 67(8): 795-800. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||