化学学报 ›› 2021, Vol. 79 ›› Issue (10): 1265-1272.DOI: 10.6023/A21060263 上一篇 下一篇
研究论文
白子昂a, 陈瑞兴a, 庞宏伟b, 王祥学b, 宋刚c, 于淑君a,*( )
)
投稿日期:2021-06-09
发布日期:2021-07-20
通讯作者:
于淑君
基金资助:
Ziang Baia, Ruixing Chena, Hongwei Pangb, Xiangxue Wangb, Gang Songc, Shujun Yua( )
)
Received:2021-06-09
Published:2021-07-20
Contact:
Shujun Yu
Supported by:文章分享
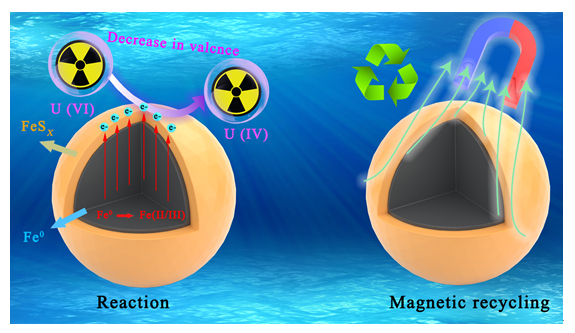
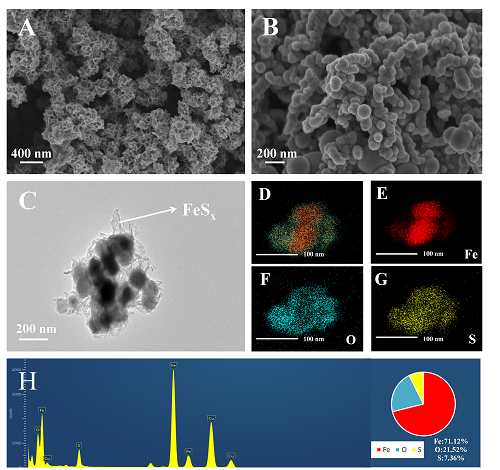
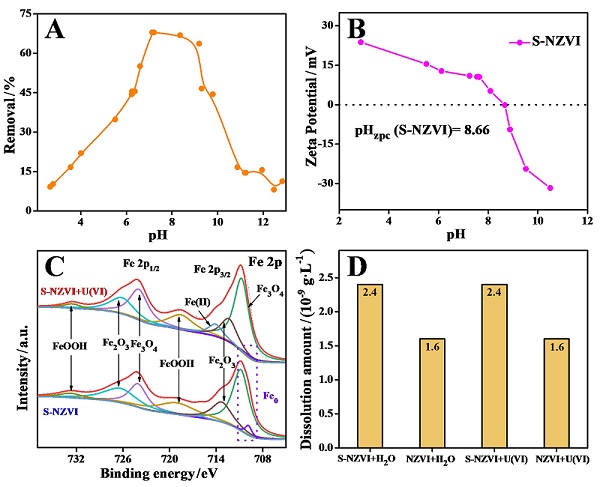
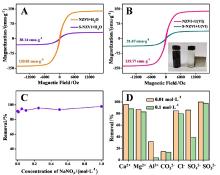
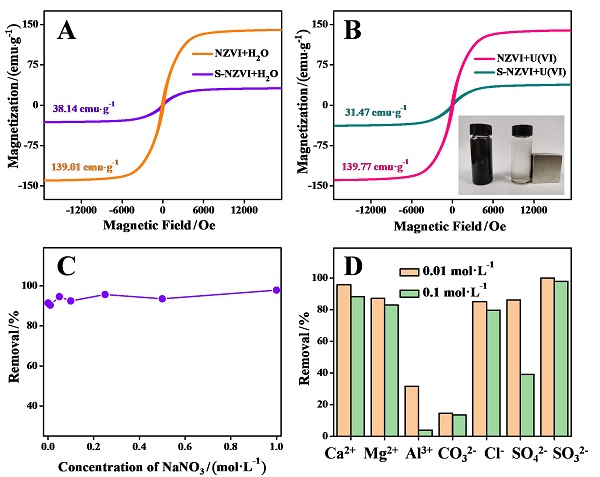
近年来, 放射性污染物铀(U(VI))在水环境中的排放对生态环境和生物健康造成严重的威胁. 本研究采用液相还原法制备了硫化纳米零价铁(S-NZVI)材料, 并将其用于水中U(VI)的去除. 首先, 我们采取了一系列的微观表征技术探究了S-NZVI的表面特征及材料特性. 结果表明, 相比于纳米零价铁(NZVI), S-NZVI颗粒不易团聚, 性质更加稳定. 随后, 通过宏观实验探究了反应时间、温度、pH、背景离子浓度等因素对S-NZVI去除U(VI)的影响. 结果表明, S-NZVI对U(VI)的最大去除量高达562.5 mg•g-1, 且在100 min内达到反应平衡. 宏观实验和X射线光电子能谱(XPS)分析表明S-NZVI对U(VI)的去除机理是吸附和氧化还原协同作用的结果. 此外, S-NZVI可以通过外加磁场从水中快速地进行分离, 便于材料再回收与利用. 综上, 本研究构筑了一种制备简单、便于回收且高效的U(VI)净化材料, 未来可能会在放射性核素的处理处置等相关工作中起到重要作用.
白子昂, 陈瑞兴, 庞宏伟, 王祥学, 宋刚, 于淑君. 硫化纳米零价铁对水中U(VI)的高效去除研究[J]. 化学学报, 2021, 79(10): 1265-1272.
Ziang Bai, Ruixing Chen, Hongwei Pang, Xiangxue Wang, Gang Song, Shujun Yu. Investigation on the Efficient Removal of U(VI) from Water by Sulfide Nanoscale Zero-valent Iron[J]. Acta Chimica Sinica, 2021, 79(10): 1265-1272.



| 温度/℃ | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
| KL/ (L•mg-1) | Qmax/ (mg•g-1) | R2 | KF/ (mg1-n•Ln•g-1) | n | R2 | ||
| 20 | 0.04 | 562.5 | 0.96 | 90.72 | 1.91 | 0.89 | |
| 25 | 0.14 | 497.9 | 0.98 | 79.98 | 1.93 | 0.92 | |
| 40 | 0.25 | 173.1 | 0.94 | 50.53 | 2.84 | 0.95 | |
| 温度/℃ | Langmuir模型 | Freundlich模型 | |||||
|---|---|---|---|---|---|---|---|
| KL/ (L•mg-1) | Qmax/ (mg•g-1) | R2 | KF/ (mg1-n•Ln•g-1) | n | R2 | ||
| 20 | 0.04 | 562.5 | 0.96 | 90.72 | 1.91 | 0.89 | |
| 25 | 0.14 | 497.9 | 0.98 | 79.98 | 1.93 | 0.92 | |
| 40 | 0.25 | 173.1 | 0.94 | 50.53 | 2.84 | 0.95 | |
| 材料 | 去除量/(mg•g-1) | 参考文献 |
|---|---|---|
| 多孔石墨型氮化碳 | 149.7 | [ |
| PEI-磁性酵母复合材料 | 173.9 | [ |
| 石墨负载纳米型零价铁 | 43.2 | [ |
| 壳聚糖/氧化石墨烯材料 | 45.0 | [ |
| 木屑季铵螯合吸附剂 | 99.7 | [ |
| 改性累托石 | 35.5 | [ |
| 向日葵秸秆 | 327.0 | [ |
| 污泥基生物炭 | 80.3 | [ |
| 羧甲基纤维素-NZVI | 203.0 | [ |
| 钙基膨润土负载NZVI | 400.0 | [ |
| 碳载零价铁 | 77.3 | [ |
| 高炉水渣负载S-NZVI | 99.8 | [ |
| 硫化纳米零价铁 | 562.5 | [本文] |
| 材料 | 去除量/(mg•g-1) | 参考文献 |
|---|---|---|
| 多孔石墨型氮化碳 | 149.7 | [ |
| PEI-磁性酵母复合材料 | 173.9 | [ |
| 石墨负载纳米型零价铁 | 43.2 | [ |
| 壳聚糖/氧化石墨烯材料 | 45.0 | [ |
| 木屑季铵螯合吸附剂 | 99.7 | [ |
| 改性累托石 | 35.5 | [ |
| 向日葵秸秆 | 327.0 | [ |
| 污泥基生物炭 | 80.3 | [ |
| 羧甲基纤维素-NZVI | 203.0 | [ |
| 钙基膨润土负载NZVI | 400.0 | [ |
| 碳载零价铁 | 77.3 | [ |
| 高炉水渣负载S-NZVI | 99.8 | [ |
| 硫化纳米零价铁 | 562.5 | [本文] |

| [1] |
Shi, W. Q.; Yuan, L. Y.; Li, Z. J.; Lan, J. H.; Zhao, Y. L.; Chai, Z. F. Radiochim. Acta 2012, 100, 727.
doi: 10.1524/ract.2012.1961 |
| [2] |
Pritee, P.; Madhurima, P.; Piyush, K. P. J. J. Nucl. Mater. 2021, 328, 89.
|
| [3] |
Shao, Y.; Yang, G. S.; Zhang, J. L.; Luo, M.; Ma, L. L.; Xu, D. D. Acta Chim. Sinica 2021, 79, 716. (in Chinese)
doi: 10.6023/A21020074 |
|
邵阳, 杨国胜, 张继龙, 罗敏, 马玲玲, 徐殿斗, 化学学报, 2021, 79, 716.)
doi: 10.6023/A21020074 |
|
| [4] |
Li, Z. N.; Sha, H. Y.; Yang, N.; Yuan, Y.; Zhu, G. S. Acta Chim. Sinica 2019, 77, 469. (in Chinese)
doi: 10.6023/A19010028 |
|
(李樟楠, 沙浩岩, 杨南, 元野, 朱广山, 化学学报, 2019, 77, 469.)
doi: 10.6023/A19010028 |
|
| [5] |
Pang, H. W.; Tang, H.; Wang, J. Q.; Wang, X. X.; Yu, S. J. Inorg. Mater. 2020, 35, 381.
|
| [6] |
Wang, N.; Pang, H. W.; Yu, S. J.; Gu, P. C.; Song, S.; Wang, H. Q.; Wang, X. K. Acta Chim. Sinica 2019, 77, 143. (in Chinese)
doi: 10.6023/A18090404 |
|
(王宁, 庞宏伟, 于淑君, 顾鹏程, 宋爽, 王宏青, 王祥科, 化学学报, 2019, 77, 143.)
doi: 10.6023/A18090404 |
|
| [7] |
Wang, X. D.; Liu, S. J.; Xu, M. Environ. Chem. 2020, 40, 1. (in Chinese)
|
|
(王煦栋, 刘思金, 徐明, 环境化学, 2020, 40, 1.)
|
|
| [8] |
Liu, X. L.; Pang, H. W.; Liu, X. W.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M. Q.; Hu, B. W.; Yang, H.; Wang, X. K. The Innovation 2021, 2, 100076.
doi: 10.1016/j.xinn.2021.100076 |
| [9] |
Chen, H. J.; Huang, S. Y.; Zhang, Z. B.; Liu, Y. H.; Wang, X. K. Acta Chim. Sinica 2017, 75, 560. (in Chinese)
doi: 10.6023/A17010039 |
|
(陈海军, 黄舒怡, 张志宾, 刘云海, 王祥科, 化学学报, 2017, 75, 560.)
doi: 10.6023/A17010039 |
|
| [10] |
Tang, J.; Feng, H. P.; Dong, H. R.; Zhang, Y.; Liu, S. S.; Zeng, G. M. Acta Chim. Sinica 2017, 75, 575. (in Chinese)
doi: 10.6023/A17020045 |
|
(汤晶, 冯浩朋, 董浩然, 章毅, 刘诗思, 曾光明, 化学学报, 2017, 75, 575.)
doi: 10.6023/A17020045 |
|
| [11] |
Li, Z. X.; Han, Y. T.; Xu, Y. Q.; Yang, Y.; Chen, J. W. Rock. Min. Anal. 2016, 35, 634. (in Chinese)
|
|
(李志雄, 韩奕彤, 徐永强, 杨洋, 陈家玮, 岩矿测试, 2016, 35, 634.)
|
|
| [12] |
Yu, S. J.; Wang, X. X.; Liu, Y. F.; Chen, Z. S.; Wu, Y. H.; Liu, Y.; Pang, H. W.; Song, G.; Chen, J. R.; Wang, X. K. Chem. Eng. J. 2019. 365, 51.
doi: 10.1016/j.cej.2019.02.024 |
| [13] |
Huang, K. Y.; Shen, Y. J.; Wang, X. Y.; Wang, X. R.; Yuan, W. Y.; Zhang, C. L.; Bai, J. F.; Wang, J. W. Environ. Eng. 2020, 38, 203. (in Chinese)
|
|
(黄开友, 申英杰, 王晓岩, 王兴润, 苑文仪, 张承龙, 白建峰, 王景伟, 环境工程, 2020, 38, 203.)
|
|
| [14] |
Pang, H. W.; Wu, Y. H.; Huang, S. Y.; Ding, C. C.; Li, S.; Wang, X. X.; Yu, S. J.; Chen, Z. S.; Song, G.; Wang, X. K. Inorg. Chem. Front. 2018, 5, 2657.
doi: 10.1039/C8QI00779A |
| [15] |
Na, L. Y.; Zhang, L. Y.; Zhang, F. J.; Hua, R. N. Mater. Rep. 2020, 34, 22030. (in Chinese)
|
|
(那立艳, 张丽影, 张凤杰, 华瑞年, 材料导报, 2020, 34, 22030.)
|
|
| [16] |
Zhao, Z. G.; Tang, X. Y. J. Henan Polytechnic University Nat. Sci. 2002, 21, 1. (in Chinese)
|
|
(赵志根, 唐修义, 焦作工学院学报(自然科学版), 2002, 21, 1.)
|
|
| [17] |
Liu, Y.; Huo, Y. Z.; Wang, X. X.; Yu, S. J.; Ai, Y. J.; Chen, Z. S.; Zhang, P.; Chen, L.; Song, G.; Alharbi, N. S.; Rabah, S. O.; Wang, X. K. J. Clean. Prod. 2021, 278, 123216.
doi: 10.1016/j.jclepro.2020.123216 |
| [18] |
Zhang, C. L.; Liu, Y.; Li, X.; Chen, H. X.; Wen, T.; Jiang, Z. H; Ai, Y. J.; Yu, S. J.; Sun, Y. B.; Tasawar, H.; Wang, X. K. Chem. Eng. J. 2018, 346, 406.
doi: 10.1016/j.cej.2018.03.186 |
| [19] |
Wu, S. Y.; Li, S. Y.; Hu, J. Y.; He, J. Q.; Wang, G. H.; Rong, L. S.; Jin, Y. Y. Acta Materiae Compositae Sinica 2020, 37, 11. (in Chinese)
|
|
(伍随意, 李仕友, 胡俊毅, 贺俊钦, 王国华, 荣丽杉, 金远远, 复合材料学报, 2020, 37, 11.)
|
|
| [20] |
Liu, X.; Li, X. Y.; Chen, Y. J.; Sang, W. X.; Chen, R. Chin. J. Nonferrous Met. 2020, 30, 1967. (in Chinese)
|
|
(刘学, 李小燕, 陈玉洁, 桑伟璇, 陈蓉, 中国有色金属学报, 2020, 30, 1967.)
|
|
| [21] |
Li, S. Y.; Shi, D. F.; Tang, Z. P.; Xie, S. B.; Liu, Y. J.; Ling, H. Acta Scientiae Circumstantiae 2017, 37, 1388. (in Chinese)
|
|
(李仕友, 史冬峰, 唐振平, 谢水波, 刘迎九, 凌辉, 环境科学学报, 2017, 37, 1388.)
|
|
| [22] |
Deng, W. J.; Zhou, S. K.; Liu, Y. J.; Zeng, G. M.; Jiang, H. H.; Kang, L.; Fang, L. Chin. J. Nonferrous Met. 2015, 25, 2604. (in Chinese)
|
|
(邓文静, 周书葵, 刘迎九, 曾光明, 江海浩, 康丽, 方良, 中国有色金属学报, 2015, 25, 2604.)
|
|
| [23] |
Guo, Y. D.; Liang, P.; Li, X. M.; Liu, M. Q. China Ceramics 2014, 50, 24. (in Chinese)
|
|
(郭亚丹, 梁平, 李效萌, 刘明清, 中国陶瓷, 2014, 50, 24.)
|
|
| [24] |
Ai, L.; Luo, X. G.; Lin, X. Y.; Mei, Q. Chem. Ind. Forest Prod. 2014, 34, 9. (in Chinese)
|
|
(艾莲, 罗学刚, 林晓艳, 梅强, 林产化学与工业, 2014, 34, 9.)
|
|
| [25] |
Mo, G. H.; Nong, H. D.; Hu, Q.; Xie, S. B.; Liu, H. J.; Zeng, T. T. Fine Chemicals 2021, 38, 395. (in Chinese)
|
|
(莫官海, 农海杜, 胡青, 谢水波, 刘红娟, 曾涛涛, 精细化工, 2021, 38, 395.)
|
|
| [26] |
I-Carmen, P.; Petru, F.; Doina, H.; Ionel, H.; Thomas, B. C.; Richard, A. C.. J. Nucl. Mater. 2013, 443, 250.
doi: 10.1016/j.jnucmat.2013.07.018 |
| [27] |
Xiong, X. H.; Chen, Q. S.; Zhou, J. W.; Liu, X. Y.; Wang, L. Y.; Huang, B.; Zhu, Y. A.; Luo, T. A. Non-metallic Minerals 2018, 41, 83. (in Chinese)
|
|
(熊小红, 陈泉水, 周佳玮, 刘星雨, 王玲钰, 黄彬, 朱业安, 罗太安, 非金属矿, 2018, 41, 83.)
|
|
| [28] |
Wang, J. Q.; Pang, H. W.; Tang, H.; Yu, S. J.; Zhu, H. T.; Wang, X. X. J. Inorg. Mater. 2020, 35, 373. (in Chinese)
|
|
(王佳琦, 庞宏伟, 唐昊, 于淑君, 朱洪涛, 王祥学, 无机材料学报, 2020, 35, 373.)
|
|
| [29] |
Sun, Q. N.; Zhang, R. B.; Deng, M. J.; Li, Y.; Wang, X. J. Environ. Sci. 2021, 42, 867. (in Chinese)
|
|
(孙秋楠, 张荣斌, 邓曼君, 李远, 王学江, 环境科学, 2021, 42, 867.)
|
|
| [30] |
Liu, Q. W.; Ding, Y. D.; Liao, Q.; Wang, H.; Zhu, X.; Zeng, F. Q. Chin. Sci. Bull. 2019, 64, 2441. (in Chinese)
doi: 10.1360/N972019-00061 |
|
(刘骐玮, 丁玉栋, 廖强, 王宏, 朱恂, 曾烽棋, 科学通报, 2019, 64, 2441.)
|
|
| [31] |
Liu, Y.; Pang, H. W.; Wang, X. X.; Yu, S. J.; Chen, Z. S.; Zhang, P.; Chen, L.; Song, G.; Alharbi, N. S.; Rabah, S. O.; Wang, X. K. Chem. Eng. J. 2021, 406, 127139.
doi: 10.1016/j.cej.2020.127139 |
| [32] |
Guo, W. J.; Liang, X. F.; Lin, D. S.; Xu, Y. M.; Wang, L.; Sun, Y. B.; Qin, X. Environ. Sci. 2013, 34, 3716. (in Chinese)
|
|
(郭文娟, 梁学峰, 林大松, 徐应明, 王林, 孙约兵, 秦旭, 环境科学, 2013, 34, 3716.)
|
|
| [33] |
Ayben, K. Appl. Radiat. Isot. 2003, 58, 713.
doi: 10.1016/S0969-8043(03)00116-7 |
| [34] |
Tan, P. L. J. Catal. 2016, 338, 21.
doi: 10.1016/j.jcat.2016.01.027 |
| [35] |
Toru, Y.; Peter, H. Appl. Surf. Sci. 2008, 254, 2441.
doi: 10.1016/j.apsusc.2007.09.063 |
| [36] |
Chen, R.; Sang, W. X.; Li, X. Y.; He, D. W.; Wang, Y.; Cao, X. G. Industrial Water Treatment 2021, 41, 66. (in Chinese)
|
|
(陈蓉, 桑伟璇, 李小燕, 何登武, 王杨, 曹小岗, 工业水处理, 2021, 41, 66.)
|
|
| [37] |
Fan, J.; Hu, Y. B.; Li, X. Y. ACS Sustain. Chem. Eng. 2018, 6, 15135.
doi: 10.1021/acssuschemeng.8b03593 |
| [38] |
Sourjay, B.; Subhasis, G. Environ. Sci. Technol. 2018, 52, 11078.
doi: 10.1021/acs.est.8b02399 |
| [39] |
Sheng, G. D.; Ahmed, A.; Wafa, S.; Shatha, M.; Sheng, J.; Wang, X. K.; Li, H.; Huang, Y. Y. Carbon 2016, 99, 123.
doi: 10.1016/j.carbon.2015.12.013 |
| [40] |
Huang, H.; Wei, Y. W.; Wang, W. Y.; Liu, M. M.; Niu, Z. R. Acta Scientiae Circumstantiae 2020, 40, 128. (in Chinese)
|
|
(黄华, 魏雨薇, 王婉悦, 刘淼淼, 牛志睿, 环境科学学报, 2020, 40, 128.)
|
|
| [41] |
Li, X. Y.; Zhang, M.; Liu, Y. B.; Li, X.; Yang, B.; Hua, R.; Liu, Y. H. Chin. J. Nonferrous Met. 2015, 25, 3505. (in Chinese)
doi: 10.1016/S1003-6326(15)63992-9 |
|
(李小燕, 张明, 刘义保, 李寻, 杨波, 花榕, 刘云海, 中国有色金属学报, 2015, 25, 3505.)
|
|
| [42] |
Yan, S.; Hua, B.; Bao, Z. Y.; Yang, J.; Liu, C. X.; Deng, B. L. Environ. Sci. Technol. Lett. 2010, 44, 7783.
doi: 10.1021/es9036308 |
| [43] |
Gao, S. M.; Wang, X. D.; Qin, L.; Luo, S.; Zhao, X.; Liu, S. S.; Wang, L. S. J. Nanjing Univ. (Nat. Sci.) 2007, 358. (in Chinese)
|
|
(高树梅, 王晓栋, 秦良, 罗斯, 赵欣, 刘树深, 王连生, 南京大学学报(自然科学版), 2007, 358.)
|
|
| [44] |
Pang, H. W.; Diao, Z. F.; Wang, X. X.; Ma, Y.; Yu, S. J.; Zhu, H. T.; Chen, Z. S.; Hu, B. W.; Chen, J. R.; Wang, X. K. Chem. Eng. J. 2019, 366, 368.
doi: 10.1016/j.cej.2019.02.098 |
| [45] |
Liang, W. Y. M.S. Thesis, Hunan University, Changsha, 2019. (in Chinese)
|
|
(梁玮瑜, 硕士论文, 湖南大学,长沙, 2019.)
|
| [1] | 林航青, 马若茹, 江怡蓝, 许木榕, 林洋彭, 杜克钊. 用于卤素捕获的材料研究进展[J]. 化学学报, 2024, 82(1): 62-74. |
| [2] | 韩玉淳, 王毅琳. 长效抗菌材料的研究现状与展望★[J]. 化学学报, 2023, 81(9): 1196-1201. |
| [3] | 刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣. 高稳定二维联咔唑sp2碳共轭共价有机框架材料用于高效电催化氧还原★[J]. 化学学报, 2023, 81(8): 884-890. |
| [4] | 曾少娟, 孙雪琦, 白银鸽, 白璐, 郑爽, 张香平, 张锁江. CO2捕集分离的功能离子液体及材料研究进展★[J]. 化学学报, 2023, 81(6): 627-645. |
| [5] | 王凯晴, 袁硕, 徐王东, 霍丹, 杨秋林, 侯庆喜, 于得海. ZIF-8@B-CNF复合气凝胶的制备及其吸附性能研究[J]. 化学学报, 2023, 81(6): 604-612. |
| [6] | 李子奇, 刘力玮, 毛承晖, 周常楷, 夏旻祺, 沈桢, 郭月, 吴强, 王喜章, 杨立军, 胡征. 钴取代多金属氧酸盐作为可溶性介质提升锂硫电池性能[J]. 化学学报, 2023, 81(6): 620-626. |
| [7] | 赵振新, 姚一琨, 陈佳骏, 牛蓉, 王晓敏. 一种高熵磷酸盐正极宿主实现高稳定性锂硫电池[J]. 化学学报, 2023, 81(5): 496-501. |
| [8] | 蒋江民, 郑欣冉, 孟雅婷, 贺文杰, 陈亚鑫, 庄全超, 袁加仁, 鞠治成, 张校刚. 氟氮共掺杂多孔碳纳米片的制备及其储钾性能研究[J]. 化学学报, 2023, 81(4): 319-327. |
| [9] | 王文涛, 赖欣婷, 闫士全, 朱雷, 姚玉元, 丁黎明. 双功能气凝胶吸附-降解协同处理染料废水[J]. 化学学报, 2023, 81(3): 222-230. |
| [10] | 郑冰, 王喆, 何静, 张姣, 戚文博, 张梦圆, 于海涛. 碱(土)金属/双层α-硼烯纳米复合体的结构和功函性质的理论研究[J]. 化学学报, 2023, 81(10): 1357-1370. |
| [11] | 马雪璐, 李蒙, 雷鸣. 三核过渡金属配合物在催化反应中的研究进展[J]. 化学学报, 2023, 81(1): 84-99. |
| [12] | 田钊炜, 达伟民, 王雷, 杨宇森, 卫敏. 第二代生物柴油制备的多相催化剂的结构设计及研究进展[J]. 化学学报, 2022, 80(9): 1322-1337. |
| [13] | 王振华, 马聪, 方萍, 徐海超, 梅天胜. 有机电化学合成的研究进展[J]. 化学学报, 2022, 80(8): 1115-1134. |
| [14] | 刘芳, 潘婷婷, 任秀蓉, 鲍卫仁, 王建成, 胡江亮. HCDs@MIL-100(Fe)吸附剂的制备及其苯吸附性能研究[J]. 化学学报, 2022, 80(7): 879-887. |
| [15] | 李晓倩, 张靖, 苏芳芳, 王德超, 姚东东, 郑亚萍. 多孔离子液体的构筑及应用[J]. 化学学报, 2022, 80(6): 848-860. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||