化学学报 ›› 2022, Vol. 80 ›› Issue (3): 303-309.DOI: 10.6023/A21120561 上一篇 下一篇
所属专题: 中国科学院青年创新促进会合辑
研究论文
戴敏a,b, 雷钢铁a,*( ), 张钊b, 李智c,d, 曹湖军b,*(
), 张钊b, 李智c,d, 曹湖军b,*( ), 陈萍b
), 陈萍b
投稿日期:2021-12-14
发布日期:2022-02-07
通讯作者:
雷钢铁, 曹湖军
作者简介:基金资助:
Min Daia,b, Gangtie Leia( ), Zhao Zhangb, Zhi Lic,d, Hujun Caob(
), Zhao Zhangb, Zhi Lic,d, Hujun Caob( ), Ping Chenb
), Ping Chenb
Received:2021-12-14
Published:2022-02-07
Contact:
Gangtie Lei, Hujun Cao
About author:Supported by:文章分享
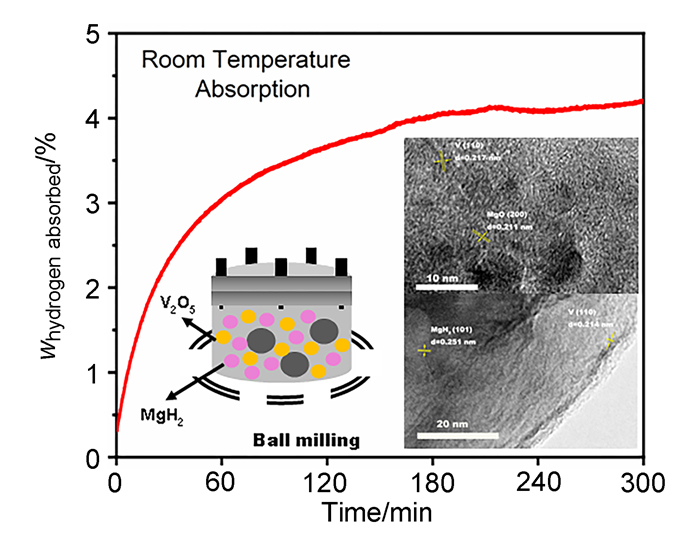
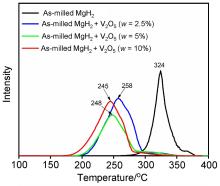
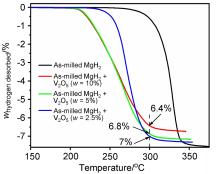
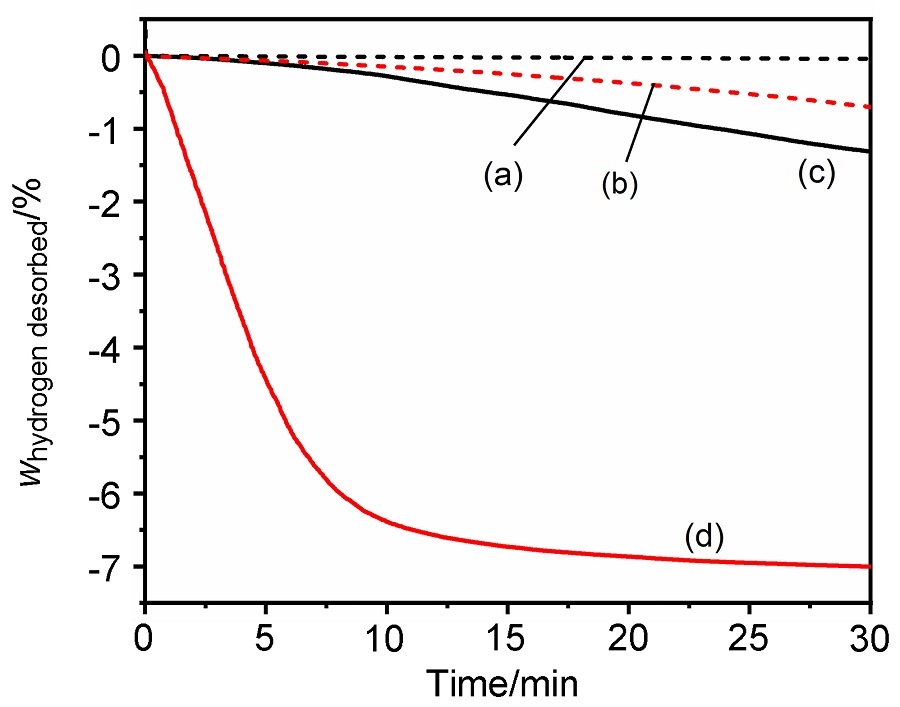
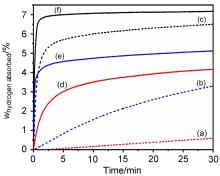
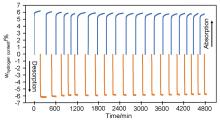
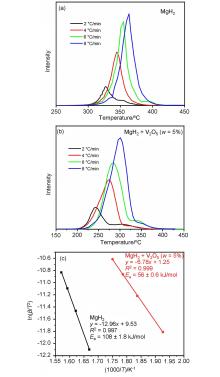
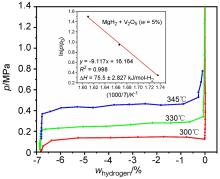
MgH2因其储氢量高、来源广及价格低廉等优点而备受关注, 但其热力学稳定(ΔH≥76 kJ/mol-H2)以及低温吸/放氢动力学缓慢等问题限制了它在氢能领域的广泛应用. 研究发现, 过渡金属氧化物能够显著改善MgH2的储氢动力学性能. 系统研究了过渡金属氧化物V2O5对MgH2储氢性能的改善作用. 与纯MgH2相比, 在MgH2中添加质量分数为5%的V2O5可以显著改善MgH2的吸/脱氢动力学性能. V2O5掺杂MgH2的起始脱氢温度降至175 ℃, 比同等条件处理的纯MgH2降低了89 ℃. 值得注意的是, V2O5掺杂的MgH2脱氢后, 在室温和3 MPa的氢压下, 30和180 min内吸收H2的质量分数分别为2.1%和3.8%. 同等氢压下, 当温度提高到300 ℃时, 该样品可在1 min内吸收H2的质量分数高达6.7%. 同时催化掺杂样品还表现出良好的循环稳定性, 20次循环后仍能维持质量分数为6.0%以上的可逆储/放氢量. 此外, V2O5改善MgH2储氢性能的反应机理也通过多种手段表征得以阐明.
戴敏, 雷钢铁, 张钊, 李智, 曹湖军, 陈萍. 五氧化二钒促进MgH2/Mg室温吸氢※[J]. 化学学报, 2022, 80(3): 303-309.
Min Dai, Gangtie Lei, Zhao Zhang, Zhi Li, Hujun Cao, Ping Chen. Room Temperature Hydrogen Absorption of V2O5 Catalyzed MgH2/Mg※[J]. Acta Chimica Sinica, 2022, 80(3): 303-309.






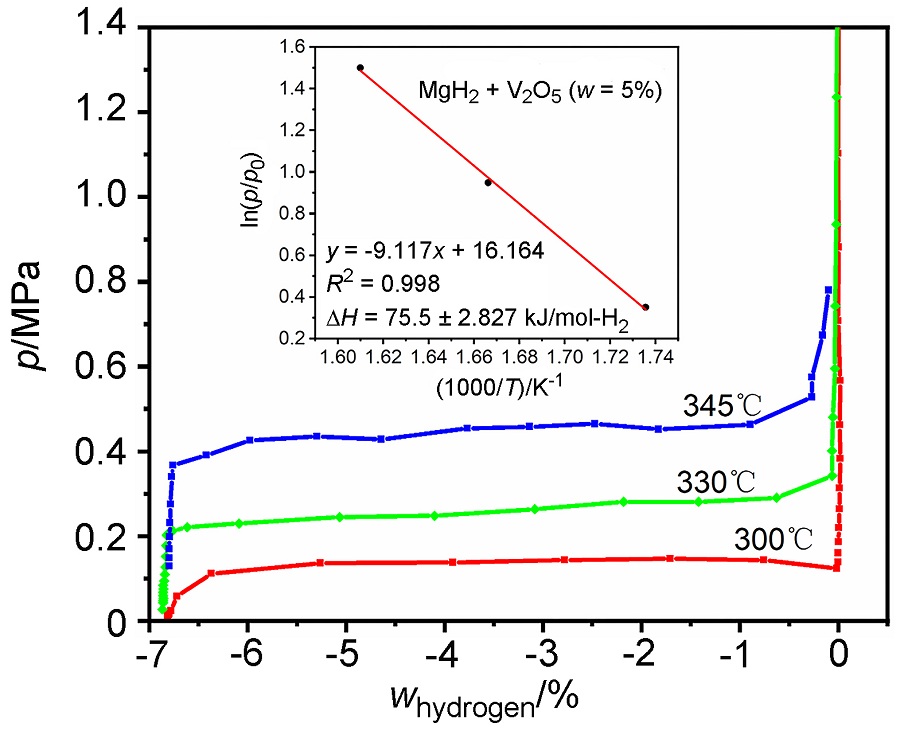
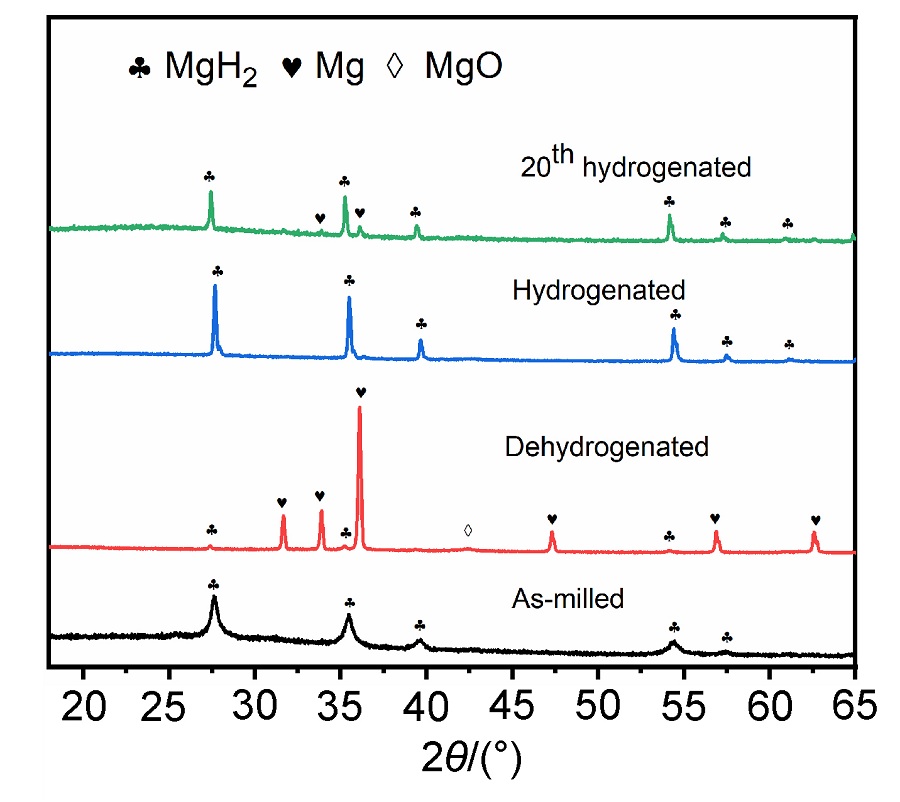



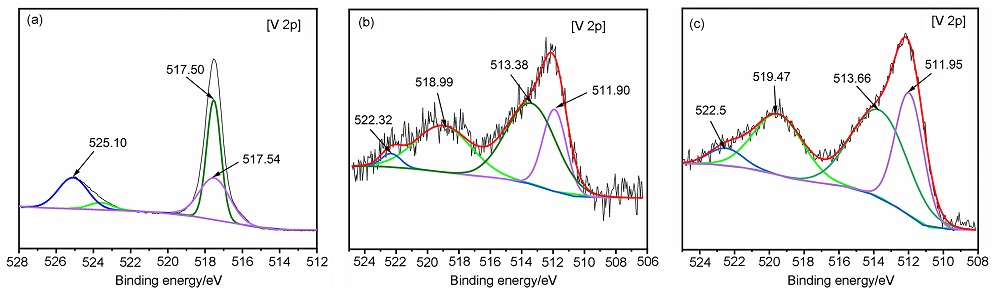
| [1] |
Lee, D.-H.; Hung, C.-P. Int. J. Hydrogen Energy 2012, 37, 15753.
doi: 10.1016/j.ijhydene.2012.02.064 |
| [2] |
Endo, N.; Goshome, K.; Tetsuhiko, M.; Segawa, Y.; Shimoda, E.; Nozu, T. Int. J. Hydrogen Energy 2021, 46, 262.
doi: 10.1016/j.ijhydene.2020.10.002 |
| [3] |
Guo, L. Chem. Eng. Des. Commun. 2021, 47, 147. (in Chinese)
doi: 10.1080/00986448608911760 |
|
(郭利, 化工设计通讯, 2021, 47, 147.)
|
|
| [4] |
Wang, F.; Harindintwali, J. D.; Yuan, Z.; Wang, M.; Wang, F.; Li, S.; Yin, Z.; Huang, L.; Fu, Y.; Li, L.; Chang, S. X.; Zhang, L.; Rinklebe, J.; Yuan, Z.; Zhu, Q.; Xiang, L.; Tsang, D. C. W.; Xu, L.; Jiang, X.; Liu, J.; Wei, N.; Kästner, M.; Zou, Y.; Ok, Y. S.; Shen, J.; Peng, D.; Zhang, W.; Barceló, D.; Zhou, Y.; Bai, Z.; Li, B.; Zhang, B.; Wei, K.; Cao, H.; Tan, Z.; Zhao, L.-B.; He, X.; Zheng, J.; Bolan, N.; Liu, X.; Huang, C.; Dietmann, S.; Luo, M.; Sun, N.; Gong, J.; Gong, Y.; Brahushi, F.; Zhang, T.; Xiao, C.; Li, X.; Chen, W.; Jiao, N.; Lehmann, J.; Zhu, Y.-G.; Jin, H.; Schäffer, A.; Tiedje, J. M.; Chen, J. M. The Innovation 2021, 2, 100180.
doi: 10.1016/j.xinn.2021.100180 |
| [5] |
Pistidda, C. Hydrogen 2021, 2, 428.
doi: 10.3390/hydrogen2040024 |
| [6] |
Xiao, G. P.; Qiao, W. J.; Zhang, L.; Qing, S. J.; Zhang, C. S.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 100. (in Chinese)
doi: 10.6023/A20080374 |
|
(肖国鹏, 乔韦军, 张磊, 庆绍军, 张财顺, 高志贤, 化学学报, 2021, 79, 100.)
doi: 10.6023/A20080374 |
|
| [7] |
Jiao, T.; Xu, X. L.; Zhang, L.; Weng, Y. Y.; Weng, Y. B.; Gao, Z. X. Acta Chim. Sinica 2021, 79, 513. (in Chinese)
doi: 10.6023/A20120562 |
|
(焦桐, 许雪莲, 张磊, 翁幼云, 翁玉冰, 高志贤, 化学学报, 2021, 79, 513.)
doi: 10.6023/A20120562 |
|
| [8] |
Chen, Y. J. Coal Chem. Ind. 2020, 43, 130. (in Chinese)
|
|
(陈英杰, 煤炭与化工, 2020, 43, 130.)
|
|
| [9] |
Hu, S. X.; Yu, Z. L.; Li, C. Y.; Wang, Z. Q.; Guo, S.; Huang, R. J.; Fang, Y. T. J. Fuel Chem. Technol. 2015, 43, 385. (in Chinese)
|
|
(胡顺轩, 余钟亮, 李春玉, 王志青, 郭帅, 黄戒介, 房倚天, 燃料化学学报, 2015, 43, 385.)
|
|
| [10] |
Zhang, Y.; Wang, S. X.; Yang, R.; Dai, T. Y.; Zhang, N.; Xi, P. X.; Yan, C. H. Acta Chim. Sinica 2020, 78, 1455. (in Chinese)
doi: 10.6023/A20070332 |
|
(张宇, 王世兴, 杨蕊, 戴腾远, 张楠, 席聘贤, 严纯华, 化学学报, 2020, 78, 1455.)
doi: 10.6023/A20070332 |
|
| [11] |
Ma, W. P.; He, Y. Y.; Liu, H. L. Acta Chim. Sinica 2021, 79, 914. (in Chinese)
doi: 10.6023/A21030121 |
|
(麻旺坪, 贺彦彦, 刘洪来, 化学学报, 2021, 79, 914.)
doi: 10.6023/A21030121 |
|
| [12] |
Feng, X.; Yang, Z. H.; CHENDe. Chem. Ind. Eng. Prog. Doi: 10.16085/j.issn.1000-6613.2021-2233. (in Chinese)
doi: 10.16085/j.issn.1000-6613.2021-2233 |
|
(冯翔, 杨朝合, CHENDe, 化工进展, Doi: 10.16085/j.issn.1000-6613.2021-2233.)
doi: 10.16085/j.issn.1000-6613.2021-2233 |
|
| [13] |
Eberle, U.; Felderhoff, M.; Schüth, F. Angew. Chem. 2009, 48, 6608.
doi: 10.1002/anie.v48:36 |
| [14] |
Schlapbach, L.; Züttel, A. Nature 2001, 414, 353.
doi: 10.1038/35104634 |
| [15] |
Zheng, J.; Wang, C.-G.; Zhou, H.; Ye, E.; Xu, J.; Li, Z.; Loh, X. J. Research 2021, 2021, 3750689.
doi: 10.34133/2021/3750689 pmid: 33623916 |
| [16] |
Lim, K. L.; Kazemian, H.; Yaakob, Z.; Daud, W. R. W. Chem. Eng. Technol. 2010, 33, 213.
doi: 10.1002/ceat.v33:2 |
| [17] |
Ma, T. X.; Gao, L. Z.; Hu, M. J.; Hu, L. W.; Wen, L. Y.; Hu, M. L. J. Funct. Mater. 2018, 49, 4001. (in Chinese)
|
|
(马通祥, 高雷章, 胡蒙均, 胡丽文, 温良英, 扈玫珑, 功能材料, 2018, 49, 4001.)
|
|
| [18] |
Sun, Y.; Shen, C.; Lai, Q.; Liu, W.; Wang, D.-W.; Aguey-Zinsou, K.-F. Energy Storage Mater. 2018, 10, 168.
|
| [19] |
Zhang, Q. Y.; Du, S. C.; Ma, Z. W.; Lin, X.; Zou, J. X.; Zhu, W.; Ren, L.; Li, Y. H. Chin. Sci. Bull. 2021, 66, 1 (in Chinese)
|
|
(张秋雨, 杜四川, 马哲文, 林羲, 邹建新, 朱文, 任莉, 李映辉, 科学通报, 2021, 66, 1.)
|
|
| [20] |
Fernández, J. F.; Sánchez, C. R. J. Alloys Compd. 2002, 340, 189.
doi: 10.1016/S0925-8388(02)00120-2 |
| [21] |
Huot, J.; Tremblay, M. L.; Schulz, R. J. Alloys Compd. 2003, 356, 603.
|
| [22] |
Bobet, J. L.; Desmoulins-Krawiec, S.; Grigorova, E.; Cansell, F.; Chevalier, B. J. Alloys Compd. 2003, 351, 217.
doi: 10.1016/S0925-8388(02)01030-7 |
| [23] |
Zhang, X.; Liu, Y.; Ren, Z.; Zhang, X.; Hu, J.; Huang, Z.; Lu, Y.; Gao, M.; Pan, H. Energy Environ. Sci. 2021, 14, 2302.
doi: 10.1039/D0EE03160G |
| [24] |
Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 292, 247.
doi: 10.1016/S0925-8388(99)00442-9 |
| [25] |
Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 315, 237.
doi: 10.1016/S0925-8388(00)01284-6 |
| [26] |
Pelletier, J. F.; Huot, J.; Sutton, M.; Schulz, R.; Sandy, A. R.; Lurio, L. B.; Mochrie, S. G. J. Phys. Rev. B 2001, 63, 052103.
doi: 10.1103/PhysRevB.63.052103 |
| [27] |
Luo, B.; Yao, Z.; Xiao, X.; Hang, Z.; Jiang, F.; Liu, M.; Chen, L. Mater. Chem. Phys. 2021, 263, 124342.
doi: 10.1016/j.matchemphys.2021.124342 |
| [28] |
Hu, S.; Zhang, H.; Yuan, Z.; Wang, Y.; Fan, G.; Fan, Y.; Liu, B. J. Alloys Compd. 2021, 881, 160571.
doi: 10.1016/j.jallcom.2021.160571 |
| [29] |
Adedeji Bolarin, J.; Zhang, Z.; Cao, H.; Li, Z.; He, T.; Chen, P. J. Phys. Chem. C 2021, 125, 19631.
doi: 10.1021/acs.jpcc.1c05560 |
| [30] |
Zhang, J.; Huang, Y. N.; Mao, C.; Long, C. G.; Shao, Y. M.; Fu, J. Q.; Peng, P. Acta Chim. Sinica 2010, 68, 2077. (in Chinese)
|
|
(张健, 黄雅妮, 毛聪, 龙春光, 邵毅敏, 付俊庆, 彭平, 化学学报, 2010, 68, 2077.)
|
|
| [31] |
Edalati, K.; Uehiro, R.; Ikeda, Y.; Li, H.-W.; Emami, H.; Filinchuk, Y.; Arita, M.; Sauvage, X.; Tanaka, I.; Akiba, E.; Horita, Z. Acta Mater. 2018, 149, 88.
doi: 10.1016/j.actamat.2018.02.033 |
| [32] |
Nyamsi, S. N.; Wu, Z.; Guo, L.; Qian, C.; Sun, L.; Xu, F.; Uesugi, H.; Yan, G.; Yang, F.; Zhang, Z. ACS Appl. Energy Mater. 2021, 4, 5973.
doi: 10.1021/acsaem.1c00819 |
| [33] |
Lu, Y.; Wang, H.; Liu, J.; Ouyang, L.; Zhu, M. J. Power Sources 2018, 396, 796.
doi: 10.1016/j.jpowsour.2018.06.060 |
| [34] |
Liao, W.; Jiang, W.; Yang, X.-S.; Wang, H.; Ouyang, L.; Zhu, M. J. Rare Earths 2021, 39, 1010.
doi: 10.1016/j.jre.2020.07.020 |
| [35] |
Kato, S.; Borgschulte, A.; Bielmann, M.; Züttel, A. Phys. Chem. Chem. Phys. 2012, 14, 8360.
doi: 10.1039/c2cp23491b |
| [36] |
Chu, H.; Qiu, S.; Sun, L.; Huot, J. Dalton Trans. 2015, 44, 16694.
doi: 10.1039/C5DT01847A |
| [37] |
Yu, H.; Bennici, S.; Auroux, A. Int. J. Hydrogen Energy 2014, 39, 11633.
doi: 10.1016/j.ijhydene.2014.05.069 |
| [38] |
Liu, H.; Lu, C.; Wang, X.; Xu, L.; Huang, X.; Wang, X.; Ning, H.; Lan, Z.; Guo, J. ACS Appl. Mater. Interfaces 2021, 13, 13235.
doi: 10.1021/acsami.0c23150 |
| [39] |
Su, W.; Zhu, Y.; Zhang, J.; Liu, Y.; Yang, Y.; Mao, Q.; Li, L. J. Alloys Compd. 2016, 669, 8.
doi: 10.1016/j.jallcom.2016.01.253 |
| [40] |
Liang, G.; Huot, J.; Boily, S.; Van Neste, A.; Schulz, R. J. Alloys Compd. 1999, 291, 295.
doi: 10.1016/S0925-8388(99)00268-6 |
| [41] |
Hanada, N.; Ichikawa, T.; Isobe, S.; Nakagawa, T.; Tokoyoda, K.; Honma, T.; Fujii, H.; Kojima, Y. J. Phys. Chem. C 2009, 113, 13450.
doi: 10.1021/jp901859f |
| [42] |
Barkhordarian, G.; Klassen, T.; Bormann, R. J. Phys. Chem. B 2006, 110, 11020.
doi: 10.1021/jp0541563 |
| [43] |
Oelerich, W.; Klassen, T.; Bormann, R. J. Alloys Compd. 2001, 322, L5.
doi: 10.1016/S0925-8388(01)01173-2 |
| [44] |
Korablov, D.; Nielsen, T. K.; Besenbacher, F.; Jensen, T. R. Powder Diffr. 2015, 30, S9.
doi: 10.1017/S0885715615000056 |
| [1] | 张静, 汤功奥, 曾誉, 王保兴, 刘力玮, 吴强, 杨立军, 王喜章, 胡征. 可溶性氧化-还原介质促进分级结构碳纳米笼的锂氧电池性能[J]. 化学学报, 2020, 78(6): 572-576. |
| [2] | 王啸, 李有彬, 杜玲玉, 高福杰, 吴强, 杨立军, 陈强, 王喜章, 胡征. 石墨烯包覆的硫填充碳纳米笼自支撑整体材料的制备及其锂硫电池性能研究[J]. 化学学报, 2018, 76(8): 627-632. |
| [3] | 陆霞, 吴仁香, 李波波, 朱云峰, 李李泉. 用于聚合物镍氢电池的新型PVA/SiO2碱性微孔聚合物电解质[J]. 化学学报, 2013, 71(03): 427-432. |
| [4] | 刘素琴,陈东洋,黄可龙,仲晓铃. MgNi-x% TiNi0.5Mn0.5 (x=10, 30, 50)贮氢合金的制备与电化学性能[J]. 化学学报, 2009, 67(6): 513-518. |
| [5] | 王新智, 李智, 王小芳, 董发昕, 王惠. 铁粉为原料催化改性铁氧化物的储氢性能[J]. 化学学报, 2008, 66(4): 437-442. |
| [6] | 王小芳, 王惠, 田勇, 董发昕, 史启祯, 文振翼. 一种新型储氢材料——改性四氧化三铁的储氢性能研究[J]. 化学学报, 2007, 65(7): 601-606. |
| [7] | 冯艳,焦丽芳,袁华堂,王一菁,刘强,刘毅. Mg0.9Ti0.1Ni1-xCox (x=0.05, 0.1, 0.15, 0.2)合金的合成及电化学性能研究[J]. 化学学报, 2006, 64(5): 423-427. |
| [8] | 赖克强,王一蕾,杨帆,孔爱国,丁旵明,单永奎. 烷基紫精/五氧化二钒插层化合物的组装、表征及磁性的研究[J]. 化学学报, 2006, 64(14): 1456-1464. |
| [9] | 廖世健,张双青,余淑文. 由蒽镁真空热解合成的活性镁的加氢性能[J]. 化学学报, 1992, 50(8): 767-771. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||