化学学报 ›› 2021, Vol. 79 ›› Issue (9): 1146-1153.DOI: 10.6023/A21040178 上一篇 下一篇
研究论文
投稿日期:2021-04-26
发布日期:2021-07-27
通讯作者:
贾桂霄
基金资助:
Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia( )
)
Received:2021-04-26
Published:2021-07-27
Contact:
Guixiao Jia
Supported by:文章分享
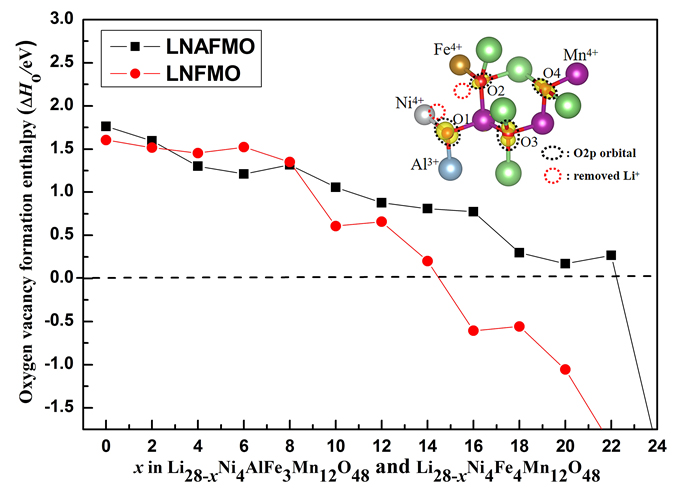
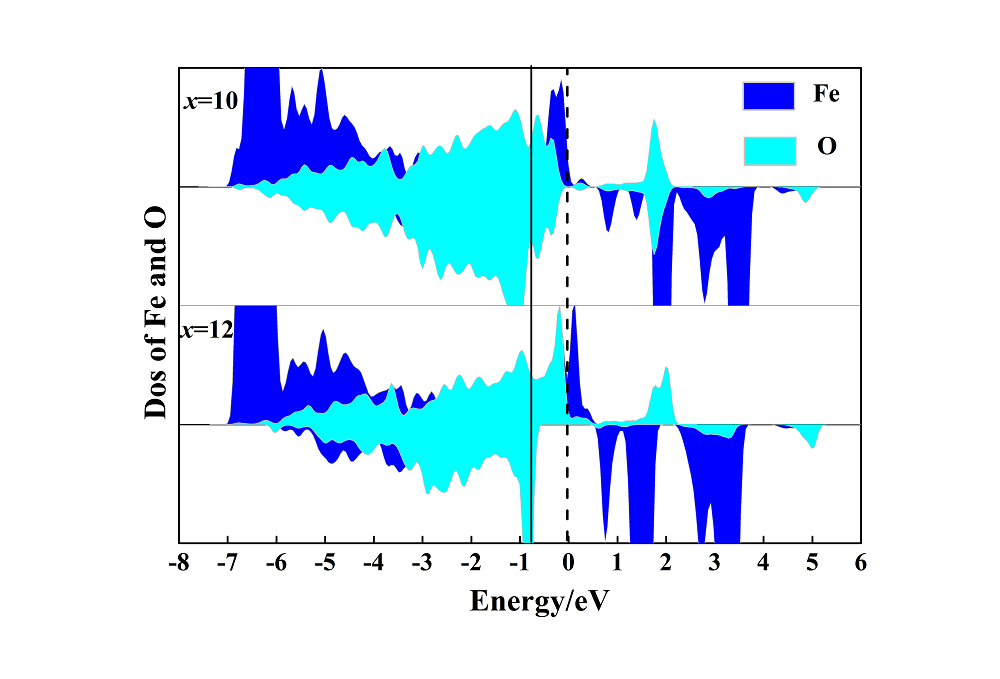
锂离子电池(LIB)正极材料比容量及结构稳定性的提高是提升电池整体性能的重要因素. 本工作选取层状无钴正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2 (LNAFMO)为研究对象, 使用GGA (generalized gradient approximation)+U (Hubbard U value)方法研究了体系在充电时几何和电子结构变化、氧释放焓、脱锂形成能和脱锂电压. 研究结果表明, 充电时LNAFMO体系首先Ni氧化, 然后Fe氧化, 最后O氧化. 与未掺杂Al的Li(Li0.17Ni0.17Fe0.17Mn0.49)O2 (LNFMO)体系不同的是, 除具有线性Li-O-Li和Fe-O-Li构型的氧离子更容易给出电子外, 具有线性Al-O-Li构型的氧离子也参与电荷补偿, 并且氧离子具有很强的活性, 这将避免参与氧化的氧离子过分集中, 有利于结构的稳定; Al的掺杂能进一步抑制氧的释放, 这将提升体系的结构稳定性和电池循环性能. 该研究为设计一种低经济成本、循环性良好、高能量密度的锂离子电池正极材料奠定了坚实的理论依据.
邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153.
Kai Qiu, Mingxia Yan, Shouwang Zhao, Shengli An, Wei Wang, Guixiao Jia. Theoretical Study on the Structural Stability and Oxygen Ion Oxidation of Al-doped Lithium-ion Battery Layered Cathode Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2[J]. Acta Chimica Sinica, 2021, 79(9): 1146-1153.


| x | a/Å | b/Å | c/Å | β/(°) | d/Å | ∆V/% | ∆HLi/eV | Ed/eV | Vave*/V | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5.04 | 4.36 | 5.10 | 109.19 | 2.05 | — | 0 | — | — | |||||
| 2 | 5.02 | 4.37 | 5.10 | 108.79 | 2.81 | 0.00 | –1.87 | –0.73 | 3.39 | |||||
| 4 | 5.00 | 4.38 | 5.10 | 108.43 | 2.85 | 0.01 | –3.91 | –0.69 | 3.30 | |||||
| 6 | 5.01 | 4.36 | 5.12 | 108.61 | 2.89 | 0.07 | –5.36 | –0.68 | 3.60 | |||||
| 8 | 5.02 | 4.35 | 5.13 | 108.35 | 2.93 | 0.48 | –7.14 | –0.66 | 3.43 | |||||
| 10 | 5.01 | 4.34 | 5.17 | 107.96 | 3.00 | 1.23 | –7.83 | –0.64 | 3.98 | |||||
| 12 | 5.01 | 4.34 | 5.21 | 108.33 | 3.06 | 0.82 | –7.77 | –0.60 | 4.36 | |||||
| 14 | 4.99 | 4.34 | 5.25 | 109.71 | 3.08 | 0.96 | –7.45 | –0.56 | 4.48 | |||||
| 16 | 4.99 | 4.33 | 5.28 | 109.61 | 3.13 | 1.34 | –7.00 | –0.51 | 4.55 | |||||
| 18 | 4.99 | 4.33 | 5.31 | 110.77 | 3.18 3.21 | 1.48 | –5.88 | –0.46 | 4.88 | |||||
| 20 | 5.02 | 4.36 | 5.36 | 112.51 | 1.45 | –4.50 | –0.43 | 5.02 | ||||||
| 22 | 4.99 | 4.30 | 5.30 | 112.20 | 3.13 | –0.65 | –5.20 | –0.51 | 3.97 | |||||
| 24 | 5.01 | 4.30 | 5.03 | 110.22 | 2.98 | –1.87 | –1.32 | –0.38 | 6.27 | |||||
| x | a/Å | b/Å | c/Å | β/(°) | d/Å | ∆V/% | ∆HLi/eV | Ed/eV | Vave*/V | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 5.04 | 4.36 | 5.10 | 109.19 | 2.05 | — | 0 | — | — | |||||
| 2 | 5.02 | 4.37 | 5.10 | 108.79 | 2.81 | 0.00 | –1.87 | –0.73 | 3.39 | |||||
| 4 | 5.00 | 4.38 | 5.10 | 108.43 | 2.85 | 0.01 | –3.91 | –0.69 | 3.30 | |||||
| 6 | 5.01 | 4.36 | 5.12 | 108.61 | 2.89 | 0.07 | –5.36 | –0.68 | 3.60 | |||||
| 8 | 5.02 | 4.35 | 5.13 | 108.35 | 2.93 | 0.48 | –7.14 | –0.66 | 3.43 | |||||
| 10 | 5.01 | 4.34 | 5.17 | 107.96 | 3.00 | 1.23 | –7.83 | –0.64 | 3.98 | |||||
| 12 | 5.01 | 4.34 | 5.21 | 108.33 | 3.06 | 0.82 | –7.77 | –0.60 | 4.36 | |||||
| 14 | 4.99 | 4.34 | 5.25 | 109.71 | 3.08 | 0.96 | –7.45 | –0.56 | 4.48 | |||||
| 16 | 4.99 | 4.33 | 5.28 | 109.61 | 3.13 | 1.34 | –7.00 | –0.51 | 4.55 | |||||
| 18 | 4.99 | 4.33 | 5.31 | 110.77 | 3.18 3.21 | 1.48 | –5.88 | –0.46 | 4.88 | |||||
| 20 | 5.02 | 4.36 | 5.36 | 112.51 | 1.45 | –4.50 | –0.43 | 5.02 | ||||||
| 22 | 4.99 | 4.30 | 5.30 | 112.20 | 3.13 | –0.65 | –5.20 | –0.51 | 3.97 | |||||
| 24 | 5.01 | 4.30 | 5.03 | 110.22 | 2.98 | –1.87 | –1.32 | –0.38 | 6.27 | |||||
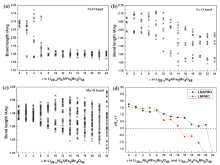

| System | M | Eb/eV | ${{\overline{E}}_{\text{b}}}$./eV | |
|---|---|---|---|---|
| LNAFMO | Fe | 1.28 | 1.29 | |
| Ni | 1.26 | |||
| Mn | 1.30 | |||
| Al | 1.30 | |||
| LNFMO | Fe | 1.15 | 1.19 | |
| Ni | 1.18 | |||
| Mn | 1.25 | |||
| System | M | Eb/eV | ${{\overline{E}}_{\text{b}}}$./eV | |
|---|---|---|---|---|
| LNAFMO | Fe | 1.28 | 1.29 | |
| Ni | 1.26 | |||
| Mn | 1.30 | |||
| Al | 1.30 | |||
| LNFMO | Fe | 1.15 | 1.19 | |
| Ni | 1.18 | |||
| Mn | 1.25 | |||



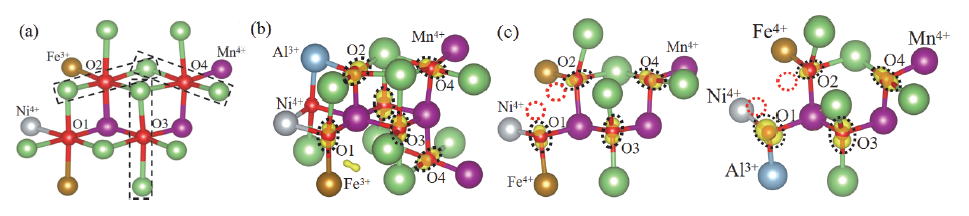
| [1] |
Huang, X. J. Materials China 2010, 29, 46. (in Chinese)
|
|
( 黄学杰, 中国材料进展, 2010, 29, 46.)
|
|
| [2] |
Xu, H.; Zhong, H. Chinese J. Inorg. Chem. 2004, 19, 497. (in Chinese)
|
|
( 许惠, 钟辉, 无机化学学报, 2004, 19, 497.)
|
|
| [3] |
Yin, D.; Daobin, M.; Borong, W.; Rui, W. Z. Appl. Energ. 2017, 195, 586.
doi: 10.1016/j.apenergy.2017.03.074 |
| [4] |
Ke, W.; Wei, Y. M.; Zhang, X. Appl. Energ. 2013, 104, 105.
doi: 10.1016/j.apenergy.2012.11.039 |
| [5] |
Yang, Z. G.; Zhang, J. L.; Kintner-Meyer, M. C. W.; Lu, X. C.; Choi, D. W.; Lemmon, J. P.; Liu, J. Chem. Rev. 2011, 111, 3577.
doi: 10.1021/cr100290v |
| [6] |
Feng, C. S.; Rui, X.; Hong, W. H. Appl. Energ. 2016, 162, 1399.
doi: 10.1016/j.apenergy.2014.12.021 |
| [7] |
Yan, J. D. Chinese J. Aeronayt. 2014, 35, 2767. (in Chinese)
|
|
( 闫金定, 航空学报, 2014, 35, 2767.)
|
|
| [8] |
Wang, L.; Gao, P. Z.; Li, D. Y.; Huang, S. T.; Xiao, H. N. Bull. Chin. Ceram. Soc. 2013, 32, 77. (in Chinese)
|
|
( 王玲, 高朋召, 李冬云, 黄诗婷, 肖汉宁, 硅酸盐通报, 2013, 32, 77.)
|
|
| [9] |
Deng, B.; Sun, W.; Wang, H.; Chen, T.; Li, X.; Qu, M.; Peng, G. Acta Chim. Sinica 2018, 76, 259. (in Chinese)
doi: 10.6023/A17110517 |
|
( 邓邦为, 孙万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂, 化学学报, 2018, 76, 259.)
doi: 10.6023/A17110517 |
|
| [10] |
Ma, C.; Lv, Y. C.; Li, H. Energy Storage Science and Technology 2014, 3, 53. (in Chinese)
|
|
( 马璨, 吕迎春, 李泓, 储能科学与技术, 2014, 3, 53.)
|
|
| [11] |
Li, H.; Zhang, L. P.; Yu, X. J. Bull. Chin. Ceram. Soc. 2012, 31, 1486. (in Chinese)
|
|
( 李恒, 张丽鹏, 于先进, 硅酸盐通报, 2012, 31, 1486.)
|
|
| [12] |
Li, W.; Tian, W. H.; Qi, L. Inorg. Chem. Ind. 2015, 47, 1. (in Chinese)
|
|
( 李卫, 田文怀, 其鲁, 无机盐工业, 2015, 47, 1.)
|
|
| [13] |
Nitta, N.; Wu, F. X.; Lee, J. T.; Yushin, G. Mater. Today 2015, 18, 252.
doi: 10.1016/j.mattod.2014.10.040 |
| [14] |
Luo, K.; Roberts, M. R.; Hao, R.; Guerrini, N.; Pickup, D. M.; Liu, Y. S.; Edstrom, K.; Guo, J. H.; Chadwick, A. V.; Duda, L. C.; Bruce, P. G. Nat. Chem. 2016, 8, 684.
doi: 10.1038/nchem.2471 |
| [15] |
Li, B.; Xia, D. G. Adv. Mater. 2017, 29, 1.
|
| [16] |
Cheng, X. L.; Wei, H. Z.; Hao, W. J.; Li, H. Y.; Si, H. N.; An, S. L.; Zhu, W. T.; Jia, G. X.; Qiu, X. P. ChemSusChem 2019, 12, 1162.
doi: 10.1002/cssc.v12.6 |
| [17] |
Zhuo, Z. Q.; Dai, K. H.; Qiao, R. M.; Wang, R.; Wu, J. P.; Liu, Y. L.; Peng, J. Y.; Chen, L. Q.; Chuang, Y. D.; Pan, F.; Shen, Z. X.; Liu, G.; Li, H.; Devereaux, T. P.; Yang, W. L. Joule 2021, 5, 975.
doi: 10.1016/j.joule.2021.02.004 |
| [18] |
Wang, Z. X.; Chen, L. Q.; Huang, X. J. Prog. Chem. 2011, 23, 284. (in Chinese)
|
|
( 王兆翔, 陈立泉, 黄学杰, 化学进展, 2011, 23, 284.)
|
|
| [19] |
Hassoun, J.; Kim, J.; Lee, D. J.; Jung, H. G.; Lee, S. M.; Sun, Y. K.; Scrosati, B. J. Power Sources 2012, 202, 308.
doi: 10.1016/j.jpowsour.2011.11.060 |
| [20] |
Ma, Q. X.; Meng, J. X.; Yang, L.; Cao, W. Chin. J. Nonferrous Met. 2013, 23, 456. (in Chinese)
doi: 10.1016/S1003-6326(13)62485-1 |
|
( 马全新, 孟军霞, 杨磊, 曹文, 中国有色金属学报, 2013, 23, 456.)
|
|
| [21] |
Li, Z.; Wang, Z.; Ban, L. Q.; Wang, J. T.; Lu, S. G. Acta Chim. Sinica 2019, 77, 1115. (in Chinese)
doi: 10.6023/A19070265 |
|
( 李钊, 王忠, 班丽卿, 王建涛, 卢世刚, 化学学报, 2019, 77, 1115.)
doi: 10.6023/A19070265 |
|
| [22] |
Gao, Y. R.; Wang, X. F.; Ma, J.; Wang, Z. X.; Chen, L. Q. Chem. Mater. 2015, 27, 3456.
doi: 10.1021/acs.chemmater.5b00875 |
| [23] |
Nakahara, K.; Tabuchi, M.; Kuroshima, S.; Toda, A.; Tanimoto, K.; Nakano, K. J. Electrochem. Soc. 2012, 159, A1398.
doi: 10.1149/2.014209jes |
| [24] |
Tabuchi, M.; Nabeshima, Y.; Takeuchi, T.; Tatsumi, K.; Imaizumi, J.; Nitta, Y. J. Power Sources 2010, 195, 834.
doi: 10.1016/j.jpowsour.2009.08.059 |
| [25] |
Laisa, C. P.; Kumar, A. K. N.; Chandrasekaran, S. S.; Murugan, P.; Lakshminarasimhan, N.; Govindaraj, R.; Ramesha, K. J. Power Sources 2016, 324, 462.
doi: 10.1016/j.jpowsour.2016.05.107 |
| [26] |
Gao, Y. R; Wang, X. F.; Ma, J.; Wang, Z. X.; Chen, L. Q. Chem. Mater. 2015, 27, 3456.
doi: 10.1021/acs.chemmater.5b00875 |
| [27] |
Zheng, J.; Gu, M.; Xiao, J.; Polzin, B. J.; Yan, P.; Chen, X.; Wang, C.; Zhang, G. Chem. Mater. 2014, 26, 6320.
doi: 10.1021/cm502071h |
| [28] |
Kang, S. H.; Johnson, C. S.; Vaughey, J. T.; Amine, K.; Thackeray, M. M. J. Electrochem. Soc. 2006, 153, A1186.
doi: 10.1149/1.2194764 |
| [29] |
Qing, R. P.; Shi, J. L.; Xiao, D. D.; Zhang, X. D.; Yin, Y. X.; Zhai, Y. B.; Gu, L.; Guo, Y. G. Adv. Energy Mater. 2016, 6, 1501914.
doi: 10.1002/aenm.201501914 |
| [30] |
Jang, Y. I.; Chiang, Y. M. Solid State Ionics 2000, 130, 53.
doi: 10.1016/S0167-2738(00)00310-6 |
| [31] |
Wang, L. Z.; Wang, H. F.; Gu, S. H.; Wang, S. X. Battery Bimonthly 2005, 2, 155. (in Chinese)
|
|
( 王力臻, 王红芳, 谷书华, 王树新, 电池, 2005, 2, 155.)
|
|
| [32] |
He, A. Z. Inorg. Chem. Ind. 2017, 49, 74 (in Chinese)
|
|
( 何爱珍, 无机盐工业, 2017, 49, 74.)
|
|
| [33] |
Ren, X. Q.; Li, D. L.; Zhao, Z. Z.; Chen, G. Q.; Zhao, K.; Kong, X. Z.; Li, T. X. Acta Chim. Sinica 2020, 78, 1268. (in Chinese)
doi: 10.6023/A20070319 |
|
( 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心, 化学学报, 2020, 78, 1268.)
doi: 10.6023/A20070319 |
|
| [34] |
Liang, Y. L. M.S. Thesis, Harbin Institute of Technology, Harbin, 2010. (in Chinese)
|
|
( 梁一林, 硕士论文, 哈尔滨工业大学, 哈尔滨, 2010.)
|
|
| [35] |
Wei, H. Z.; Cheng, X. L.; Fan, H. W.; Shan, Q.; An, S. L.; Qiu, X. P.; Jia, G. X. ChemSusChem 2019, 12, 2471.
|
| [36] |
Wei, H. Z. M.S. Thesis, Inner Mongolia University of Science and Technology, Baotou, 2019. (in Chinese)
|
|
( 卫河转, 硕士论文, 内蒙古科技大学, 包头, 2019.)
|
|
| [37] |
Cao, T.; Shi, C.; Zhao, N.; He, C. N.; Li, J. J.; Liu, E. Z. J. Phys. Chem. C 2015, 119, 28749.
doi: 10.1021/acs.jpcc.5b09948 |
| [38] |
Luo, K.; Roberts, M. R.; Guerrini, N.; Tapia-Ruiz, N.; Hao, R.; Massel, F.; Pickup, D. M,; Ramos, S.; Liu, Y. S.; Guo, J. H.; Chadwick, A. V.; Duda, L. C.; Bruce, P. G. J. Am. Chem. Soc. 2016, 138, 11211.
doi: 10.1021/jacs.6b05111 |
| [39] |
Seo, D. H.; Lee, J.; Urban, A.; Malik, R.; Kang, S.; Ceder, G. Nat. Chem. 2016, 8, 692.
doi: 10.1038/nchem.2524 |
| [40] |
Kresse, G.; Furthmüller J. Comp. Mater. Sci. 1996, 6, 15.
doi: 10.1016/0927-0256(96)00008-0 |
| [41] |
Song, L. B.; Li, A. X.; Xiao, Z. L.; Chi, Z. Z.; Cao, Z. J. Chem. Ind. Eng. 2019, 70, 2051. (in Chinese)
|
|
( 宋刘斌, 黎安娴, 肖忠良, 池振振, 曹忠, 化工学报, 2019, 70, 2051.)
|
|
| [42] |
Zhang, Y. J. M.S. Thesis, Shandong University, Jinan, 2013. (in Chinese)
|
|
( 张雍家, 硕士论文, 山东大学, 济南, 2013.)
|
|
| [43] |
Xu, B.; Wang, Z. Met. Funct. Mater. 2012, 19, 40. (in Chinese)
|
|
( 徐本刚, 王忠, 金属功能材料, 2012, 19, 40.)
|
|
| [44] |
Dudarev, S. L.; Botton, G. A.; Savrasov, S. Y. Phys. Rev. B 1998, 57, 1505.
doi: 10.1103/PhysRevB.57.1505 |
| [45] |
Xu, Y. H.; Yi, G. P.; Zuo, J. P. Prog. Chem. 2008, 11, 1827. (in Chinese)
|
|
( 徐宇虹, 尹鸽平, 左朋建, 化学进展, 2008, 11, 1827.)
|
|
| [46] |
De Dompablo, M. E. A. Y.; Ceder, G.; J. Power. Sources 2003, 119-121, 654.
doi: 10.1016/S0378-7753(03)00199-X |
| [1] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [2] | 张玉莹, 蔡潇, 胡维刚, 李光俊, 祝艳. Pd和Hg共掺杂的金属纳米团簇HgPdAu23(PET)18★[J]. 化学学报, 2023, 81(7): 703-708. |
| [3] | 刘振宇, 饶俊峰, 祝守加, 王兵洋, 余帆, 冯全友, 解令海. 溶液加工型自主体热活化延迟荧光材料的研究进展[J]. 化学学报, 2023, 81(7): 820-835. |
| [4] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [5] | 蒋江民, 郑欣冉, 孟雅婷, 贺文杰, 陈亚鑫, 庄全超, 袁加仁, 鞠治成, 张校刚. 氟氮共掺杂多孔碳纳米片的制备及其储钾性能研究[J]. 化学学报, 2023, 81(4): 319-327. |
| [6] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [7] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [8] | 王娟, 肖华敏, 谢丁, 郭元茹, 潘清江. 铜掺杂与氮化碳复合氧化锌材料结构和二氧化氮气体传感性质的密度泛函理论计算[J]. 化学学报, 2023, 81(11): 1493-1499. |
| [9] | 刘蕊, 孟彬, 胡俊丽, 刘俊. 具有超低LUMO/HOMO能级的稠环芳烃分子★[J]. 化学学报, 2023, 81(10): 1295-1300. |
| [10] | 张国强, 霍京浩, 王鑫, 郭守武. 基于P掺杂TiO2/C纳米管负极的高性能锂离子电容器[J]. 化学学报, 2023, 81(1): 6-13. |
| [11] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [12] | 李小娟, 叶梓瑜, 谢书涵, 王永净, 王永好, 吕源财, 林春香. 氮氯共掺杂多孔碳活化过一硫酸盐降解苯酚的性能及机理研究[J]. 化学学报, 2022, 80(9): 1238-1249. |
| [13] | 齐志豪, 高福杰, 周常楷, 曾誉, 吴强, 杨立军, 王喜章, 胡征. 氮掺杂碳纳米笼固载钌纳米粒子的费托合成性能[J]. 化学学报, 2022, 80(8): 1100-1105. |
| [14] | 闫绍兵, 焦龙, 何传新, 江海龙. ZIF-67/石墨烯复合物衍生的氮掺杂碳限域Co纳米颗粒用于高效电催化氧还原[J]. 化学学报, 2022, 80(8): 1084-1090. |
| [15] | 李海茹, 张层, 李思殿. 碱土金属Ben (n=1~3)对B12团簇结构的调控研究[J]. 化学学报, 2022, 80(7): 888-895. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||