Acta Chimica Sinica ›› 2019, Vol. 77 ›› Issue (11): 1177-1183.DOI: 10.6023/A19070276 Previous Articles Next Articles
Article
投稿日期:2019-07-26
发布日期:2019-09-24
通讯作者:
于淑君
E-mail:sjyu@ncepu.edu.cn
基金资助:
Shi Leia, Pang Hongweia, Wang Xiangxueb, Zhang Panb, Yu Shujuna*( )
)
Received:2019-07-26
Published:2019-09-24
Contact:
Yu Shujun
E-mail:sjyu@ncepu.edu.cn
Share
Shi Lei, Pang Hongwei, Wang Xiangxue, Zhang Pan, Yu Shujun. Study on the Migration and Transformation Mechanism of Graphene Oxide in Aqueous Solutions[J]. Acta Chimica Sinica, 2019, 77(11): 1177-1183.
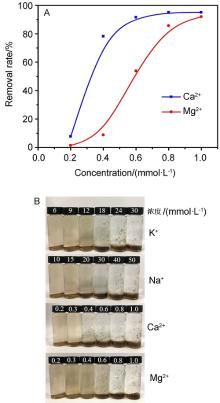
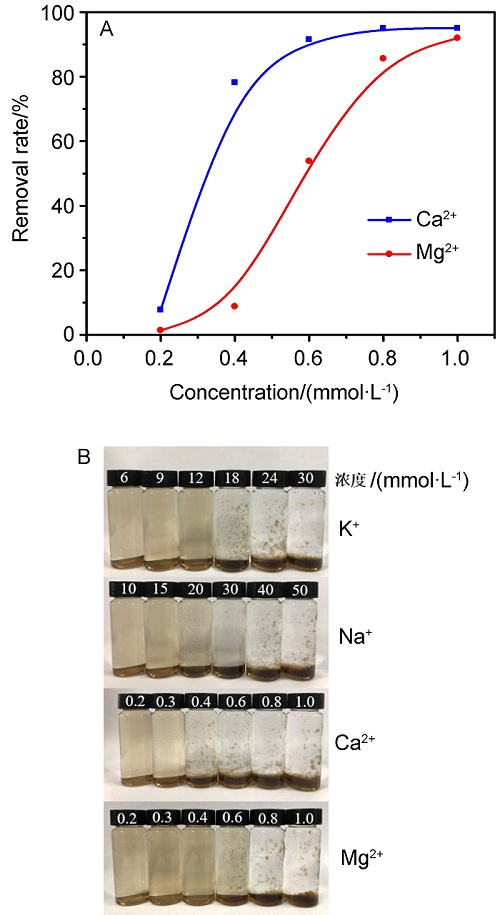
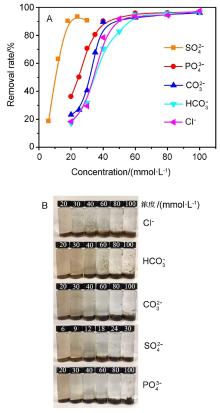
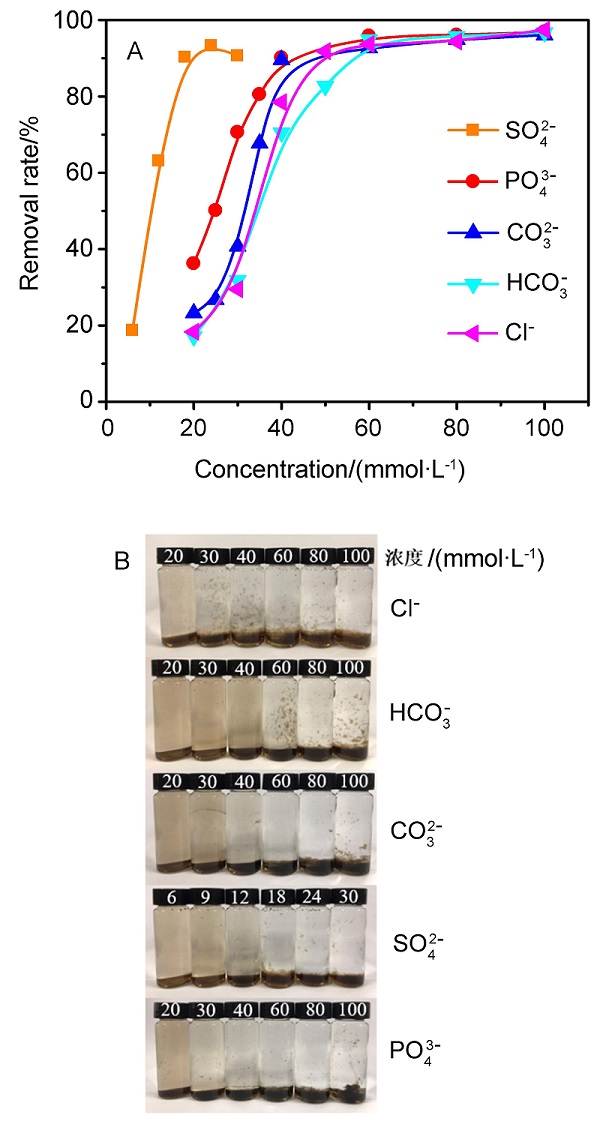


| [1] |
Loh K. P.; Bao Q. L.; Ang P. K.; Yang J. X. J. Mater. Chem. 2010, 20, 2277.
doi: 10.1039/b920539j |
| [2] |
Dreyer D. R.; Park S. J.; Bielawski C. W.; Ruoff R. S. Chem. Soc. Rev. 2010, 39, 228.
doi: 10.1039/B917103G |
| [3] |
Chen D.; Feng H.; Li J. Chem. Rev. 2012, 112, 6027.
doi: 10.1021/cr300115g |
| [4] |
Stoller M. D.; Park S. J.; Zhu Y. W.; An j.; Ruoff R. S. Nano Lett. 2008, 8, 3498.
doi: 10.1021/nl802558y |
| [5] |
Zhao K. L.; Hao Y.; Zhu M.; Cheng G. S. Acta Chim. Sinica. 2018, 76, 168.
doi: 10.3866/PKU.WHXB201707111 |
|
赵 克丽; 郝 莹; 朱 墨; 程 国胜 化学学报 2018, 76, 168.
doi: 10.3866/PKU.WHXB201707111 |
|
| [6] |
Zhang S. W.; Zhang J.; Wu S. D.; lv W.; Kang F. Y.; Yang Q. H. Acta. Chim. Sinica. 2017, 75, 163.
doi: 10.11862/CJIC.2017.023 |
|
张 思伟; 张 俊; 吴 思达; 吕 伟; 康 飞宇; 杨 全红 化学学报 2017, 75, 163.
doi: 10.11862/CJIC.2017.023 |
|
| [7] |
Zhong G. Y.; Wang H. J.; Yu H.; Peng F. Acta Chim. Sinica. 2017, 75, 943
doi: 10.6023/A17040183 |
|
钟 国玉; 王 红娟; 余 皓; 彭 峰 化学学报 2017, 75, 943
doi: 10.6023/A17040183 |
|
| [8] |
Wang X.; Li Y. B.; Du L. Y.; Gao F. J.; Wu Q.; Yang L. J.; Chen Q.; Wang X. J.; Hu Z. Acta Chim. Sinica. 2018, 76, 627.
doi: 10.11862/CJIC.2018.081 |
|
王 啸; 李 有彬; 杜 玲玉; 高 福杰; 吴 强; 杨 立军; 陈 强; 王 喜章; 胡 征 化学学报 2018, 76, 627.
doi: 10.11862/CJIC.2018.081 |
|
| [9] |
Lu J. H.; Tan S. Z.; Zhu Y. Q.; Li W.; Chen T. X.; Wang Y.; Liu C. Acta Chim. Sinica. 2019, 77, 253
doi: 10.6023/A18100433 |
|
卢 静荷; 谭 淑珍; 朱 雨清; 李 伟; 陈 天啸; 王 瑶; 刘 陈 化学学报 2019, 77, 253
doi: 10.6023/A18100433 |
|
| [10] |
Ma W. H.; Chang Y. Z.; Han G. Y.; Xiao Y. M.; Fu D. Y.; Chang Y. H. Chinese J. Chem. 2017, 35, 1844.
doi: 10.1002/cjoc.201700398 |
| [11] |
Yang X. L.; Cai H. Y.; Bao M. Y.; Yu J. Q.; Lu J. R.; Li Y. M. Chinese J. Chem. 2017, 35, 1549.
doi: 10.1002/cjoc.201700202 |
| [12] |
Li M. Y.; Liu R. Q.; Han G. Y.; Tian Y. N.; Chang Y. Z.; Xiao Y. M. Chinese J. Chem. 2017, 35, 1405.
doi: 10.1002/cjoc.201700061 |
| [13] |
Song C. Y.; Sun X.; Ye K.; Zhu K.; Cheng H.; Yan J.; Cao D. X.; Wang G. L. Acta. Chim. Sinica. 2017, 75, 1003
doi: 10.6023/A17070298 |
|
宋 聪颖; 孙 逊; 叶 克; 朱 凯; 程 魁; 闫 俊; 曹 殿学; 王 贵领 化学学报 2017, 75, 1003
doi: 10.6023/A17070298 |
|
| [14] |
Liao K. H.; Lin Y. S.; Macosko C. W.; Haynes C. L. ACS Appl. Mater. Interfaces 2011, 3, 2607.
doi: 10.1021/am200428v |
| [15] |
Gao Y.; Chen K.; Ren X. M.; Ahmed A.; Tasawar H.; Chen C. L. Environ. Sci. Technol. 2018, 52, 12208.
doi: 10.1021/acs.est.8b02234 |
| [16] |
Gao Y; Wu J. C.; Ren X. M.; Tan X. L. Tasawar H.; Ahmed A.; Cheng C.; Chen C. L. Environ. Sci.:Nano 2017, 4, 1016.
doi: 10.1039/C7EN00052A |
| [17] |
Gao Y.; Ren X. M.; Wu J. C.; Tasawar H.; Ahmed A.; Cheng C.; Chen C. l. Environ. Sci.:Nano 2018, 5, 362.
doi: 10.1039/C7EN01012E |
| [18] |
Wang J.; Yao W.; Gu P. C.; Yu S. J.; Wang X. X.; Du Y.; Wang H. Q.; Chen Z. S.; Hayat T.; Wang X. K. Cellulose 2016, 24, 85
doi: 10.1007/s10570-016-1117-5 |
| [19] |
Vallabani N. V. S.; Mittal S.; Shukla R. K.; Pandey A. K.; Dhakate S. R.; Pasricha R.; Dhawan A. J. Biomed. Nanotechnol. 2011, 7, 106.
doi: 10.1166/jbn.2011.1224 |
| [20] |
Akhavan O.; Ghaderi E. ACS Nano 2010, 4, 5731.
doi: 10.1021/nn101390x |
| [21] |
Hu J.; Zhang C. X.; Jiang L.; Fang S. D.; Zhang X. D.; Wang X. K.; Meng Y. D. J. Power Sources 2014, 248, 831.
doi: 10.1016/j.jpowsour.2013.09.099 |
| [22] |
Zaghouane-Boudiaf H.; Boutahala M.; Arab L. Chem. Eng. J. 2012, 187, 142.
doi: 10.1016/j.cej.2012.01.112 |
| [23] |
Wang J.; Wang X. X.; Tan L. Q.; Chen Y. T.; Hayat T.; Hu J.; Alsaedi A.; Ahmad B.; Guo W.; Wang X. K. Chem. Eng. J. 2016, 297, 106.
doi: 10.1016/j.cej.2016.04.012 |
| [24] |
Meunier N.; Drogui P.; Montane C.; Hausler R.; Mercier G.; Blais J. F. J. Hazard. Mater. 2006, 137, 581.
doi: 10.1016/j.jhazmat.2006.02.050 |
| [25] |
Qiang S. R.; Wang M. Y.; Liang J. J.; Zhao X. L.; Fan Q. H.; Geng R. Y.; Luo D. X.; Li Z. B.; Zhang L. Mater. Chem. Phys. 2020, 239, 122016.
doi: 10.1016/j.matchemphys.2019.122016 |
| [26] |
Yang K. J.; Chen B. L.; Zhu X. Y.; Xing B. S. Environ. Sci. Technol. 2016, 50, 11066.
doi: 10.1021/acs.est.6b04235 |
| [27] |
Chowdhury I.; Mansukhani N. D.; Guiney L. M.; Hersam M. C.; Bouchard D. Environ. Sci. Technol. 2015, 49, 10886.
doi: 10.1021/acs.est.5b01866 |
| [28] |
Zeng Z. Y.; Wang Y. L.; Zhou Q. B.; Yang K.; Lin D. H. Environ. Pollut. 2019, 250, 366.
doi: 10.1016/j.envpol.2019.03.112 |
| [29] |
Zhao J.; Liu F. F.; Wang Z. Y.; Cao X. S.; Xing B. S. Environ. Sci. Technol. 2015, 49, 2849.
doi: 10.1021/es505605w |
| [30] |
Liang J. J.; Li P.; Zhao X. L.; Liu Z. Y.; Fan Q. H.; Li Z.; Wang D. Q. Nanoscale 2018, 3, 1383
doi: 10.1039/c0nr00814a |
| [31] |
Park C. M.; Chu K. H.; Heo J.; Her N.; Jang M.; Son A.; Yoon Y. J. Hazard. Mater. 2016, 309, 133.
doi: 10.1016/j.jhazmat.2016.02.006 |
| [32] | Li N.; Ma J. Z.; Bao Y. Chem. Res. 2009, 20, 98 |
| 李 娜; 马 建中; 鲍 艳 化学研究 2009, 20, 98 | |
| [33] |
Huang G. X.; Guo H. Y.; Zhao J.; Liu Y. H.; Xing B. S. Water Res. 2012, 102, 313
doi: 10.1016/j.watres.2016.06.050 |
| [34] |
Anirudhan T. S.; Ramachandran M. Proc. Saf. Environ. Prot. 2015, 95, 215.
doi: 10.1016/j.psep.2015.03.003 |
| [35] |
Chowdhury I.; Duch M. C.; Mansukhani N. D.; Hersam M. C.; Bouchard D. Environ. Sci. Technol. 2013, 47, 6288.
doi: 10.1021/es400483k |
| [36] |
Wu L.; Liu L.; Gao B.; Muñoz-Carpena R.; Zhang M.; Chen H.; Zhou Z. H.; Wang H. Langmuir 2013, 29, 15174.
doi: 10.1021/la404134x |
| [37] |
Ma J. C.; Dougherty D. A. Chem. Rev. 1997, 97, 1303.
doi: 10.1021/cr9603744 |
| [38] |
Trivedi P.; Axe L.; Dyer J. Colloids Surf. A 2001, 191, 107.
doi: 10.1016/S0927-7757(01)00768-3 |
| [39] |
Raza G.; Amjad M.; Kaur I.; Wen D. Environ. Sci. Technol. 2016, 50, 8462.
doi: 10.1021/acs.est.5b05746 |
| [40] |
Volkov A. G.; Paula D. W.; Dreamer D. W. Bioelectrochem. Bioenerg. 1997, 42, 153.
doi: 10.1016/S0302-4598(96)05097-0 |
| [41] |
Tansel B.; Sager J.; Rector T.; Garland J.; Strayer R. F.; Levine L. F.; Roberts M.; Hummerick M.; Bauer J. Sep. Purif. Technol. 2006, 51, 40.
doi: 10.1016/j.seppur.2005.12.020 |
| [42] |
Abdelmeguid A. E.; Aboelfetoh E. F.; Ebeid E. M. Chemosphere 2017, 181, 738.
doi: 10.1016/j.chemosphere.2017.04.137 |
| [43] |
Tahir S. S.; Rauf N. Chemosphere 2006, 63, 1842.
doi: 10.1016/j.chemosphere.2005.10.033 |
| [44] |
Hummers W. S.; Offeman R. E. J. Am. Chem. Soc. 1958, 80, 1339.
doi: 10.1021/ja01539a017 |
| [1] | Lingyue Yang, Yunting Li, Chao Shu. Research Progress on Sultine [J]. Acta Chimica Sinica, 2024, 82(2): 171-189. |
| [2] | Wen He, Bo Wang, Hanjun Feng, Xiangru Kong, Tao Li, Rui Xiao. Research Progress of CO2 Capture and Membrane Separation by Pebax Based Materials [J]. Acta Chimica Sinica, 2024, 82(2): 226-241. |
| [3] | Ruxin Zeng, Peng R. Chen. RNA-Binding Proteome Analysis and Functional Explorations★ [J]. Acta Chimica Sinica, 2024, 82(1): 53-61. |
| [4] | Chen Li, Xiao Si, Jinbo Li, Yan Zhang. Chemical Modification and Delivery System of Small Interfering RNA Drugs★ [J]. Acta Chimica Sinica, 2023, 81(9): 1240-1254. |
| [5] | Huagao Wang, Qunfeng Cheng. Recent Advances in the Nacre-inspired Layered Polymer Nanocomposites by Ice Templating Technique★ [J]. Acta Chimica Sinica, 2023, 81(9): 1231-1239. |
| [6] | Duanda Wang, Xinyi Shen, Yongyang Song, Shutao Wang. Application Progress of Emerging Janus Particles for Oil-Water Separation★ [J]. Acta Chimica Sinica, 2023, 81(9): 1187-1195. |
| [7] | Lefei Yu, Xing-Qi Yao, Jianbo Wang. Recent Advance of Diazo Compounds in Polymer Synthesis★ [J]. Acta Chimica Sinica, 2023, 81(8): 1015-1029. |
| [8] | Shaoyan Gan, Shengyu Zhong, Liting Wang, Lei Shi. Synthesis and Application of Organic Hypervalent Bromine Reagents [J]. Acta Chimica Sinica, 2023, 81(8): 1030-1042. |
| [9] | Yongxue Li, Yu Liu. Supramolecular Secondary Assembly Based on Amphiphilic Calix[4]arenes and Its Biological Applications★ [J]. Acta Chimica Sinica, 2023, 81(8): 928-936. |
| [10] | Wei Hou, Yancai Yao, Lizhi Zhang. Advances in Electrochemical Reductive Removal of Oxyanions in Water★ [J]. Acta Chimica Sinica, 2023, 81(8): 979-989. |
| [11] | Zhixiang Yuan, Hao Zhang, Sijia Hu, Botao Zhang, Jianjun Zhang, Guanglei Cui. Research Progress of Ion-initiated in situ Generated Solid Polymer Electrolytes for High-safety Lithium Batteries★ [J]. Acta Chimica Sinica, 2023, 81(8): 1064-1080. |
| [12] | Na Yang, Jianzhong Ma, Jiabo Shi, Xu Guo. Organic Modification of Layered Double Hydroxides and Its Applications [J]. Acta Chimica Sinica, 2023, 81(2): 207-216. |
| [13] | Xuan Zhang, Jun Xiong, Wang Zhang. High Performance Blue Perovskite Light Emitting Diode Realized by Poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) Modification [J]. Acta Chimica Sinica, 2023, 81(12): 1695-1700. |
| [14] | Shanshan Jin, Sinong Wang, Hongdong Zhang, Yuliang Yang. A Study on the Comprehensive Evaluation of Chinese Handmade Paper Deacidification Effectiveness Based on Life Expectancy★ [J]. Acta Chimica Sinica, 2023, 81(12): 1681-1686. |
| [15] | Baomin Yang, Shuitao Zhang, Xian Dong, Guiping Qin, Yubo Jiang. Advances in the Modifications of 4-Monosubstituted 1,2,3-Triazoles [J]. Acta Chimica Sinica, 2023, 81(11): 1577-1589. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||