有机化学 ›› 2022, Vol. 42 ›› Issue (11): 3668-3683.DOI: 10.6023/cjoc202205049 上一篇 下一篇
研究论文
张涛a, 卫海沅a, 马雯a, 李张媛a, 胡盼盼a, 周楠茜b, 贺建超a, 李婷a, 苏明明a, 白素平a,*( )
)
收稿日期:2022-05-28
修回日期:2022-08-01
发布日期:2022-08-17
通讯作者:
白素平
基金资助:
Tao Zhanga, Haiyuan Weia, Wen Maa, Zhangyuan Lia, Panpan Hua, Nanqian Zhoub, Jianchao Hea, Ting Lia, Mingming Sua, Suping Baia( )
)
Received:2022-05-28
Revised:2022-08-01
Published:2022-08-17
Contact:
Suping Bai
Supported by:文章分享

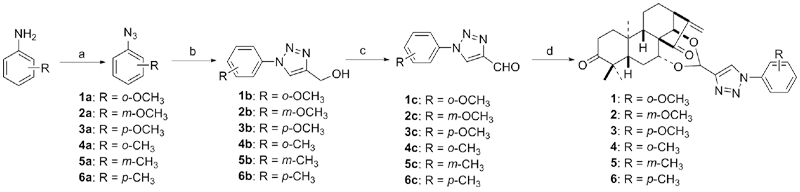
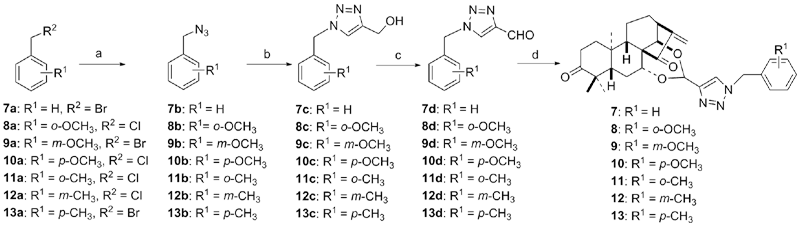
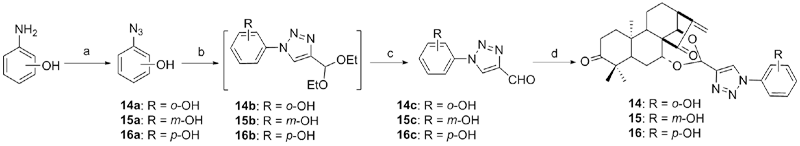
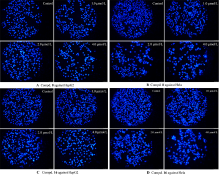
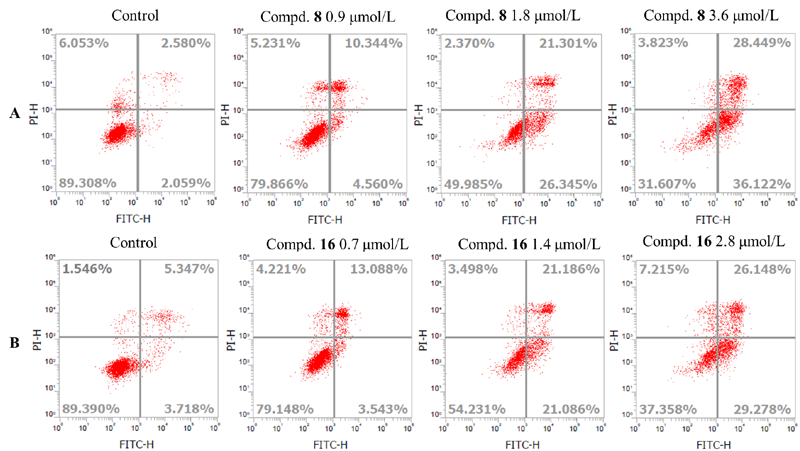
设计并合成了一系列新型的天然产物蓝萼甲素(Glaucocalyxin A, GLA)-1,2,3-三氮唑类衍生物, 并评估该系列衍生物对HepG2、NCI-H460、JEG-3、K562、HL-60和Hela等六种人肿瘤细胞株的抗增殖活性. 结果表明大多数化合物具有很强的抗增殖作用, 其中一些化合物的活性明显优于GLA. 其中(3S,3aR,3a1R,6aR,11aR)-5-(1-(4-羟基苯基)-1H-1,2,3-三唑-4-基)-8,8,11a-三甲基-13-亚甲基十氢-1H-3,3a1-乙吩并[1,10-de][1,3]二噁-9,12(2H)-二酮(16)对HL-60细胞株表现出最强的抑制作用(IC50=0.25 μmol•L-1), 其活性比阳性药阿霉素强6.9倍, 比蓝萼甲素强25.8倍. 这些结果表明在保留D环上α,β-不饱和酮药效基团的前提下, 间羟基或对羟基苯基取代的1,2,3-三氮唑缩醛结构片段的引入是提高蓝萼甲素抗肿瘤活性的有效途径. 细胞凋亡形态学和流式细胞仪测定表明蓝萼甲素-1,2,3-三氮唑类衍生物具有诱导肿瘤细胞凋亡的作用.
张涛, 卫海沅, 马雯, 李张媛, 胡盼盼, 周楠茜, 贺建超, 李婷, 苏明明, 白素平. 新型蓝萼甲素-1,2,3-三氮唑类衍生物的合成与抗增殖活性研究[J]. 有机化学, 2022, 42(11): 3668-3683.
Tao Zhang, Haiyuan Wei, Wen Ma, Zhangyuan Li, Panpan Hu, Nanqian Zhou, Jianchao He, Ting Li, Mingming Su, Suping Bai. Synthesis and Antiproliferative Activity Evaluation of Novel Glaucocalyxin A-1,2,3-Triazole Derivatives[J]. Chinese Journal of Organic Chemistry, 2022, 42(11): 3668-3683.


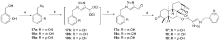
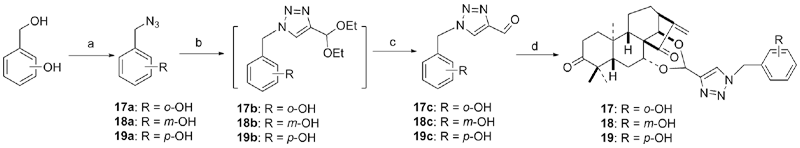
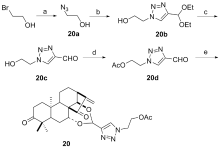
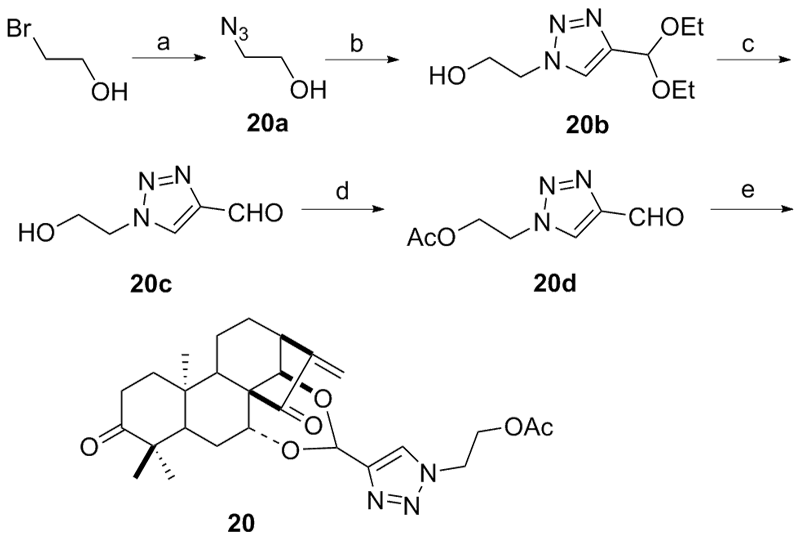
| Compd. | HepG2 | NCI-H460 | JEG-3 | K562 | HL-60 | Hela |
|---|---|---|---|---|---|---|
| 1 | 5.78±0.10 | 8.30±0.38 | 5.15±0.26 | 8.28±0.15 | 4.52±0.17 | 5.85±0.61 |
| 2 | 21.57±0.39 | 13.46±0.61 | 3.22±0.25 | 14.52±0.19 | 2.29±0.10 | 15.12±0.59 |
| 3 | 30.38±0.18 | 80.34±2.96 | 5.32±0.30 | 60.62±0.62 | 24.05±2.46 | 16.39±0.23 |
| 4 | 2.89±0.01 | 21.71±0.17 | 4.15±0.04 | 9.03±0.06 | 1.98±0.13 | 2.77±0.06 |
| 5 | 25.27±2.06 | 31.87±0.16 | 14.94±1.90 | 28.71±2.16 | 14.75±0.35 | 14.19±0.97 |
| 6 | >100 | >100 | >100 | >100 | 29.23±0.29 | 72.13±2.40 |
| 7 | 13.69±0.13 | 21.34±1.03 | 9.51±0.15 | 32.00±0.38 | 14.28±1.51 | 12.32±0.21 |
| 8 | 1.85±0.28 | 4.66±0.09 | 2.53±0.02 | 5.04±0.26 | 1.97±0.06 | 1.59±0.09 |
| 9 | 12.23±1.06 | 29.33±0.59 | 5.50±0.04 | 19.69±0.24 | 11.55±1.32 | 7.88±0.13 |
| 10 | 3.03±0.14 | 4.70±0.05 | 2.86±0.07 | 5.40±0.40 | 2.40±0.11 | 3.28±0.06 |
| 11 | 5.83±0.14 | 9.68±0.19 | 2.93±0.17 | 13.77±0.27 | 3.22±0.19 | 5.56±0.57 |
| 12 | 8.05±0.31 | 18.44±0.55 | 1.66±0.03 | 18.29±0.94 | 12.37±0.50 | 16.27±0.61 |
| 13 | 5.45±0.24 | 13.09±1.02 | 12.49±0.23 | 18.30±0.55 | 5.96±0.42 | 7.45±0.12 |
| 14 | 16.42±0.45 | 13.27±0.28 | 5.07±0.05 | 67.11±0.91 | 1.18±0.02 | 2.15±0.18 |
| 15 | 2.53±0.14 | 24.52±0.26 | 6.21±0.10 | 17.05±0.76 | 0.36±0.02 | 6.78±0.10 |
| 16 | 1.43±0.04 | 5.56±0.06 | 1.18±0.05 | 5.08±0.22 | 0.25±0.01 | 2.85±0.03 |
| 17 | 18.19±1.11 | 16.61±1.00 | 18.39±0.53 | >100 | 4.73±0.36 | 10.60±0.45 |
| 18 | 4.49±0.08 | 2.85±0.07 | 1.47±0.25 | 3.77±0.10 | 1.16±0.12 | 3.00±0.14 |
| 19 | 12.16±0.64 | 7.56±0.11 | 20.18±0.32 | 14.14±0.88 | 2.53±0.12 | 5.06±0.22 |
| 20 | 11.98±0.14 | 10.87±0.84 | 9.92±0.82 | 16.35±0.54 | 4.75±0.03 | 4.64±0.10 |
| 21 | 21.88±1.01 | 51.64±0.37 | 19.59±0.38 | 81.4±4.61 | >100 | 29.61±0.84 |
| 22 | 6.81±0.53 | 4.92±0.17 | 6.40±0.18 | 16.03±0.45 | 3.47±0.05 | 4.27±0.11 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 0.29±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |
| Compd. | HepG2 | NCI-H460 | JEG-3 | K562 | HL-60 | Hela |
|---|---|---|---|---|---|---|
| 1 | 5.78±0.10 | 8.30±0.38 | 5.15±0.26 | 8.28±0.15 | 4.52±0.17 | 5.85±0.61 |
| 2 | 21.57±0.39 | 13.46±0.61 | 3.22±0.25 | 14.52±0.19 | 2.29±0.10 | 15.12±0.59 |
| 3 | 30.38±0.18 | 80.34±2.96 | 5.32±0.30 | 60.62±0.62 | 24.05±2.46 | 16.39±0.23 |
| 4 | 2.89±0.01 | 21.71±0.17 | 4.15±0.04 | 9.03±0.06 | 1.98±0.13 | 2.77±0.06 |
| 5 | 25.27±2.06 | 31.87±0.16 | 14.94±1.90 | 28.71±2.16 | 14.75±0.35 | 14.19±0.97 |
| 6 | >100 | >100 | >100 | >100 | 29.23±0.29 | 72.13±2.40 |
| 7 | 13.69±0.13 | 21.34±1.03 | 9.51±0.15 | 32.00±0.38 | 14.28±1.51 | 12.32±0.21 |
| 8 | 1.85±0.28 | 4.66±0.09 | 2.53±0.02 | 5.04±0.26 | 1.97±0.06 | 1.59±0.09 |
| 9 | 12.23±1.06 | 29.33±0.59 | 5.50±0.04 | 19.69±0.24 | 11.55±1.32 | 7.88±0.13 |
| 10 | 3.03±0.14 | 4.70±0.05 | 2.86±0.07 | 5.40±0.40 | 2.40±0.11 | 3.28±0.06 |
| 11 | 5.83±0.14 | 9.68±0.19 | 2.93±0.17 | 13.77±0.27 | 3.22±0.19 | 5.56±0.57 |
| 12 | 8.05±0.31 | 18.44±0.55 | 1.66±0.03 | 18.29±0.94 | 12.37±0.50 | 16.27±0.61 |
| 13 | 5.45±0.24 | 13.09±1.02 | 12.49±0.23 | 18.30±0.55 | 5.96±0.42 | 7.45±0.12 |
| 14 | 16.42±0.45 | 13.27±0.28 | 5.07±0.05 | 67.11±0.91 | 1.18±0.02 | 2.15±0.18 |
| 15 | 2.53±0.14 | 24.52±0.26 | 6.21±0.10 | 17.05±0.76 | 0.36±0.02 | 6.78±0.10 |
| 16 | 1.43±0.04 | 5.56±0.06 | 1.18±0.05 | 5.08±0.22 | 0.25±0.01 | 2.85±0.03 |
| 17 | 18.19±1.11 | 16.61±1.00 | 18.39±0.53 | >100 | 4.73±0.36 | 10.60±0.45 |
| 18 | 4.49±0.08 | 2.85±0.07 | 1.47±0.25 | 3.77±0.10 | 1.16±0.12 | 3.00±0.14 |
| 19 | 12.16±0.64 | 7.56±0.11 | 20.18±0.32 | 14.14±0.88 | 2.53±0.12 | 5.06±0.22 |
| 20 | 11.98±0.14 | 10.87±0.84 | 9.92±0.82 | 16.35±0.54 | 4.75±0.03 | 4.64±0.10 |
| 21 | 21.88±1.01 | 51.64±0.37 | 19.59±0.38 | 81.4±4.61 | >100 | 29.61±0.84 |
| 22 | 6.81±0.53 | 4.92±0.17 | 6.40±0.18 | 16.03±0.45 | 3.47±0.05 | 4.27±0.11 |
| GLA | 3.94±0.07 | 8.99±0.18 | 4.25±0.08 | 7.20±0.17 | 6.46±0.05 | 8.19±1.96 |
| Adriamycin | 0.50±0.01 | 0.29±0.02 | 0.52±0.02 | 5.15±0.11 | 1.73±0.02 | 0.69±0.01 |


| [1] |
Newman, D. J.; Cragg, G. M. J. Nat. Prod. 2020, 83, 770.
doi: 10.1021/acs.jnatprod.9b01285 pmid: 32162523 |
| [2] |
Atanasov, A. G.; Zotchev, S. B.; Dirsch, V. M.; Orhan, I. E.; Banach, M.; Rollinger, J. M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E. A.; Majeed, M.; Bishayee, A.; Bochkov, V.; Bonn, G. K.; Braidy, N.; Bucar, F.; Cifuentes, A.; D’Onofrio, G.; Bodkin, M.; Diederich, M.; Dinkova-Kostova, A. T.; Efferth, T.; El Bairi, K.; Arkells, N.; Fan, T.-P.; Fiebich, B. L.; Freissmuth, M.; Georgiev, M. I.; Gibbons, S.; Godfrey, K. M.; Gruber, C. W.; Heer, J.; Huber, L. A.; Ibanez, E.; Kijjoa, A.; Kiss, A. K.; Lu, A.; Macias, F. A.; Miller, M. J. S.; Mocan, A.; Müller, R.; Nicoletti, F.; Perry, G.; Pittalà, V.; Rastrelli, L.; Ristow, M.; Russo, G. L.; Silva, A. S.; Schuster, D.; Sheridan, H.; Skalicka-Woźniak, K.; Skaltsounis, L.; Sobarzo- Sánchez, E.; Bredt, D. S.; Stuppner, H.; Sureda, A.; Tzvetkov, N. T.; Vacca, R. A.; Aggarwal, B. B.; Battino, M.; Giampieri, F.; Wink, M.; Wolfender, J.-L.; Xiao, J.; Yeung, A. W. K.; Lizard, G.; Popp, M. A.; Heinrich, M.; Berindan-Neagoe, I.; Stadler, M.; Daglia, M.; Verpoorte, R.; Supuran, C. T. Nat. Rev. Drug Discovery 2021, 20, 200.
doi: 10.1038/s41573-020-00114-z |
| [3] |
Xiang, Z.; Wu, X.; Liu, X.; Jin, Y. Nat. Prod. Res. 2014, 28, 2221.
doi: 10.1080/14786419.2014.934235 |
| [4] |
Li, D.; Han, T.; Liao, J.; Hu, X.; Xu, S.; Tian, K.; Gu, X.; Cheng, K.; Li, Z.; Hua, H.; Xu, J. Int. J. Mol. Sci. 2016, 17, 1395.
doi: 10.3390/ijms17091395 |
| [5] |
Wang, M.; Li, H.; Xu, F.; Gao, X.; Li, J.; Xu, S.; Zhang, D.; Wu, X.; Xu, J.; Hua, H.; Li, D. Eur. J. Med. Chem. 2018, 156, 885.
doi: 10.1016/j.ejmech.2018.07.052 |
| [6] |
Nagashima, F.; Kondoh, M.; Fujii, M.; Takaoka, S.; Watanabe, Y.; Asakawa, Y. Tetrahedron 2005, 61, 4531.
doi: 10.1016/j.tet.2005.03.010 |
| [7] |
Huang, S.-X.; Zhao, Q.-S.; Xu, G.; Xiao, W.-L.; Li, R.-T.; Hou, A.-J.; Peng, S.-L.; Ding, L.-S.; Sun, H.-D. J. Nat. Prod. 2005, 68, 1758.
doi: 10.1021/np050342o |
| [8] |
Li, X.; Xiao, W.; Pu, J.; Ban, L.; Shen, Y.; Weng, Z.; Li, S.; Sun, H. Phytochemistry 2006, 67, 1336.
doi: 10.1016/j.phytochem.2006.05.002 |
| [9] |
Chen, S.; Liu, J.; Zhang, H. J. Huazhong Univ. Sci. Technol., Med. Sci. 2009, 29, 659.
doi: 10.1016/0277-9536(89)90186-X |
| [10] |
Liang, H.-J.; Zhang, Y.-X.; Hai, G.-F.; Bai, S.-P.; Yuan, Y.-L.; Ye, D.-D.; Zhou, N.-Q. Planta Med. 2012, 78, 589.
doi: 10.1055/s-0031-1298265 |
| [11] |
Zhang, J.-X.; Han, Q.-B.; Zhao, A.-H.; Sun, H.-D. Fitoterapia 2003, 74, 435.
pmid: 12837357 |
| [12] |
Zhao, Y.; Niu, X.-M.; Qian, L.-P.; Liu, Z.-Y.; Zhao, Q.-S. Eur. J. Med. Chem. 2007, 42, 494.
pmid: 17189663 |
| [13] |
Liu, G.; Ding, L.; Yang, Y.; Yang, H.; Yang, Q.; Wang, H. Res. Chem. Intermed. 2006, 32, 787.
doi: 10.1163/156856706778606543 |
| [14] |
Zhang, T.; Li, N. X.; Zhou, N. Q.; Ma, W.; Wei, H. Y.; Zhang, B. X.; Chen, L. H.; Hai, G. F.; Duan, Y. C.; Bai, S. P. Chin. J. Org. Chem. 2021, 41, 2393. (in Chinese)
doi: 10.6023/cjoc202101058 |
|
( 张涛, 李念先, 周楠茜, 马雯, 卫海沅, 张冰欣, 陈亮辉, 海广范, 段迎超, 白素平, 有机化学 2021, 41, 2393.)
doi: 10.6023/cjoc202101058 |
|
| [15] |
Chen, J.; Zhang, W.; Pan, C.; Fan, J.; Zhong, X.; Tang, S. Life Sci. 2021, 271, 119185.
doi: 10.1016/j.lfs.2021.119185 |
| [16] |
Wang, X.; He, M.-J.; Chen, X.-J.; Bai, Y.-T.; Zhou, G. J. Ethnopharmacol. 2022, 290, 115100.
doi: 10.1016/j.jep.2022.115100 |
| [17] |
Ren, L.; Wang, J.; Chen, G. Drug Delivery 2019, 26, 309.
doi: 10.1080/10717544.2019.1568623 |
| [18] |
Boechat, N.; Ferreira, V. F.; Ferreira, S. B.; Ferreira, M. d. L. G.; da Silva, F. d. C.; Bastos, M. M.; Costa, M. d. S.; Lourenço, M. C. S.; Pinto, A. C.; Krettli, A. U.; Aguiar, A. C.; Teixeira, B. M.; da Silva, N. V.; Martins, P. R. C.; Bezerra, F. A. F. M.; Camilo, A. L. S.; da Silva, G. P.; Costa, C. C. P. J. Med. Chem. 2011, 54, 5988.
doi: 10.1021/jm2003624 pmid: 21776985 |
| [19] |
Bozorov, K.; Zhao, J.; Aisa, H. A. Bioorg. Med. Chem. 2019, 27, 3511.
|
| [20] |
Xu, Z.; Zhao, S.-J.; Liu, Y. Eur. J. Med. Chem. 2019, 183, 111700.
doi: 10.1016/j.ejmech.2019.111700 |
| [21] |
Ke, Y.; Wang, W.; Zhao, L.-F.; Liang, J.-J.; Liu, Y.; Zhang, X.; Feng, K.; Liu, H.-M. Bioorg. Med. Chem. 2018, 26, 4761.
doi: 10.1016/j.bmc.2017.11.005 |
| [22] |
Ke, Y.; Liang, J.-J.; Hou, R.-J.; Li, M.-M.; Zhao, L.-F.; Wang, W.; Liu, Y.; Xie, H.; Yang, R.-H.; Hu, T.-X.; Wang, J.-Y.; Liu, H.-M. Eur. J. Med. Chem. 2018, 157, 1249.
doi: 10.1016/j.ejmech.2018.08.056 |
| [23] |
Andreeva, O. V.; Garifullin, B. F.; Sharipova, R. R.; Strobykina, I. Y.; Sapunova, A. S.; Voloshina, A. D.; Belenok, M. G.; Dobrynin, A. B.; Khabibulina, L. R.; Kataev, V. E. J. Nat. Prod. 2020, 83, 2367.
doi: 10.1021/acs.jnatprod.0c00134 pmid: 32786882 |
| [24] |
Liang, L.; Astruc, D. Coord. Chem. Rev. 2011, 255, 2933.
doi: 10.1016/j.ccr.2011.06.028 |
| [25] |
Sun, S.; Wu, P. J. Phys. Chem. A 2010, 114, 8331.
doi: 10.1021/jp105034m |
| [26] |
Li, H.-Q.; Yang, J.; Ma, S.; Qiao, C. Bioorg. Med. Chem. 2012, 20, 4194.
doi: 10.1016/j.bmc.2012.05.079 |
| [27] |
Bai, S.-P.; Li, S.-H.; Xu, J.-B.; Peng, X.; Sai, K.; Chu, W.; Tu, Z.; Zeng, C.; Mach, R. H. J. Med. Chem. 2014, 57, 4239.
doi: 10.1021/jm5001453 |
| [28] |
Bertrand, H. C.; Schaap, M.; Baird, L.; Georgakopoulos, N. D.; Fowkes, A.; Thiollier, C.; Kachi, H.; Dinkova-Kostova, A. T.; Wells, G. J. Med. Chem. 2015, 58, 7186.
doi: 10.1021/acs.jmedchem.5b00602 pmid: 26348784 |
| [29] |
Manandhar, E.; Broome, J. H.; Myrick, J.; Lagrone, W.; Cragg, P. J.; Wallace, K. J. Chem. Commun. 2011, 47, 8796.
doi: 10.1039/c1cc13286e |
| [30] |
Colombano, G.; Albani, C.; Ottonello, G.; Ribeiro, A.; Scarpelli, R.; Tarozzo, G.; Daglian, J.; Jung, K.-M.; Piomelli, D.; Bandiera, T. ChemMedChem 2015, 10, 380.
doi: 10.1002/cmdc.201402374 |
| [31] |
Sánchez-Sixto, C.; Prazeres, V. F. V.; Castedo, L.; Suh, S. W.; Lamb, H.; Hawkins, A. R.; Cañada, F. J.; Jiménez-Barbero, J.; González-Bello, C. ChemMedChem 2008, 3, 756.
doi: 10.1002/cmdc.200700307 pmid: 18200648 |
| [32] |
Pirali, T.; Gatti, S.; Di Brisco, R.; Tacchi, S.; Zaninetti, R.; Brunelli, E.; Massarotti, A.; Sorba, G.; Canonico, P. L.; Moro, L.; Genazzani, A. A.; Tron, G. C.; Billington, R. A. ChemMedChem 2007, 2, 437.
doi: 10.1002/cmdc.200600192 |
| [33] |
Zhang, Q.; Takacs, J. M. Org. Lett. 2008, 10, 545.
doi: 10.1021/ol702890s pmid: 18189407 |
| [34] |
Park, K. D.; Morieux, P.; Salomé, C.; Cotten, S. W.; Reamtong, O.; Eyers, C.; Gaskell, S. J.; Stables, J. P.; Liu, R.; Kohn, H. J. Med. Chem. 2009, 52, 6897.
doi: 10.1021/jm9012054 pmid: 19795888 |
| [1] | 孙泽人, 翟冰新, 何光超, 沈慧, 陈琳雅, 张杉, 邹毅, 朱启华, 徐云根. 新型1,2,3-三氮唑类衍生物的合成及抗炎活性研究[J]. 有机化学, 2023, 43(6): 2143-2155. |
| [2] | 纪健, 刘进华, 管丛, 陈绪文, 赵芸, 刘顺英. 原位生成的磺酸催化N-磺酰基-1,2,3-三氮唑与醇偶联高区域选择性合成N2-取代1,2,3-三氮唑[J]. 有机化学, 2023, 43(3): 1168-1176. |
| [3] | 王妮, 郑姿君, 贾小苹, 赵梦圆, 王亚蕾, 周臣, 王志佳, 肖泽霖, 刘宏民, 可钰. 济源冬凌草甲素衍生物作为潜在抗肿瘤药物的合成及药理学活性研究[J]. 有机化学, 2023, 43(2): 646-659. |
| [4] | 罗云, 高谕康, 燕鹏程, 朱伟明. 浅蓝霉素H的发酵优化与浅蓝苷K的化学合成[J]. 有机化学, 2022, 42(9): 2840-2849. |
| [5] | 高潮, 司晓杰, 池玲玲, 王浩, 戴洪林, 刘丽敏, 汪正捷, 张洋, 王涛, 周耀传, 郑甲信, 可钰, 刘宏民, 张秋荣. 含苯甲醚结构的2,4,5,6-四取代嘧啶衍生物的合成及抗增殖活性研究[J]. 有机化学, 2022, 42(6): 1677-1686. |
| [6] | 楚治良, 陈晖娟, 单帅, 王晓娜, 高春芳, 渠桂荣, 刘忠于, 郭海明. 一步法合成1,2,4-三氮唑[3,4-i]嘌呤类化合物[J]. 有机化学, 2022, 42(5): 1551-1556. |
| [7] | 高潮, 张玉桐, 池玲玲, 王浩, 马家婕, 毕梦鑫, 戴洪林, 司晓杰, 刘丽敏, 张洋, 郑甲信, 可钰, 刘宏民, 张秋荣. 新型2,4,6-三取代嘧啶衍生物的合成及抗增殖活性评价[J]. 有机化学, 2022, 42(11): 3824-3834. |
| [8] | 毕晶晶, 孙潇潇, 高松, 陈长坡, 张贵生. 铜催化的2H-1,2,3-三氮唑的绿色制备[J]. 有机化学, 2021, 41(7): 2760-2766. |
| [9] | 郑茜茜, 刘云云, 万结平. 烯胺调控下和对甲苯磺酰叠氮在纯水介质中的无金属环化反应合成1,2,3-三氮唑[J]. 有机化学, 2021, 41(7): 2700-2706. |
| [10] | 赵志恒, 李鸣, 周娅琴, 何永辉, 张丽珠, 李干鹏, 谷利军. 电化学脱氢[3+2]环化反应合成取代的1,2,4-三氮唑衍生物[J]. 有机化学, 2021, 41(6): 2476-2484. |
| [11] | 张涛, 李念先, 周楠茜, 马雯, 卫海沅, 张冰欣, 陈亮辉, 海广范, 段迎超, 白素平. 新型蓝萼甲素-噻唑类衍生物的设计、合成与生物学评价[J]. 有机化学, 2021, 41(6): 2393-2400. |
| [12] | 孙雨佳, 王紫薇, 王岩, 许同绣, 田克情, 张萍. 含三氮唑结构的1,5-苯并硫氮杂䓬的合成及抑菌活性[J]. 有机化学, 2021, 41(6): 2361-2373. |
| [13] | 姚阳意, 任朝丽, 陈丽, 钟良坤, 许天明, 谭成侠. 含芳基三氮唑结构的3-乙砜基吡啶类化合物的合成及杀虫活性研究[J]. 有机化学, 2021, 41(5): 2055-2062. |
| [14] | 刘娜, 郭思岐, 刘俊芳, 陈彦韬, 徐晓明, 张静, 康亚青, 罗成, 陈示洁, 陈华. 连氨基脲链的三氮唑并噻二唑类DOT1L抑制剂的设计、合成及活性[J]. 有机化学, 2020, 40(8): 2450-2459. |
| [15] | 徐晓明, 郭思岐, 张静, 陈彦韬, 康亚青, 刘娜, 刘俊芳, 罗成, 陈示洁, 陈华. 三氮唑并噻二唑类DOT1L抑制剂的结构修饰及活性[J]. 有机化学, 2020, 40(5): 1345-1354. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||