有机化学 ›› 2023, Vol. 43 ›› Issue (2): 646-659.DOI: 10.6023/cjoc202207033 上一篇 下一篇
研究论文
王妮a,b, 郑姿君a,b, 贾小苹a,b, 赵梦圆a,b, 王亚蕾a,b, 周臣a,b, 王志佳a,b, 肖泽霖c, 刘宏民a,b,*( ), 可钰a,b,*(
), 可钰a,b,*( )
)
收稿日期:2022-07-03
修回日期:2022-10-23
发布日期:2022-11-07
基金资助:
Ni Wanga,b, Zijun Zhenga,b, Xiaoping Jiaa,b, Mengyuan Zhaoa,b, Yalei Wanga,b, Chen Zhoua,b, Zhijia Wanga,b, Zelin Xiaoc, Hongmin Liua,b( ), Yu Kea,b(
), Yu Kea,b( )
)
Received:2022-07-03
Revised:2022-10-23
Published:2022-11-07
Contact:
*E-mail: Supported by:文章分享

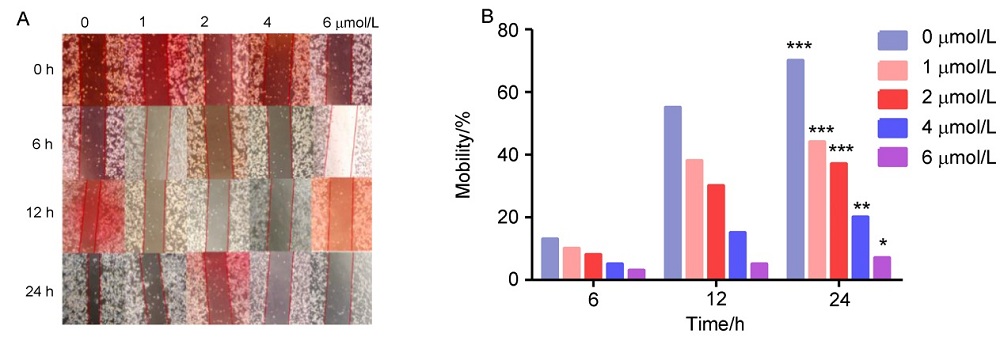
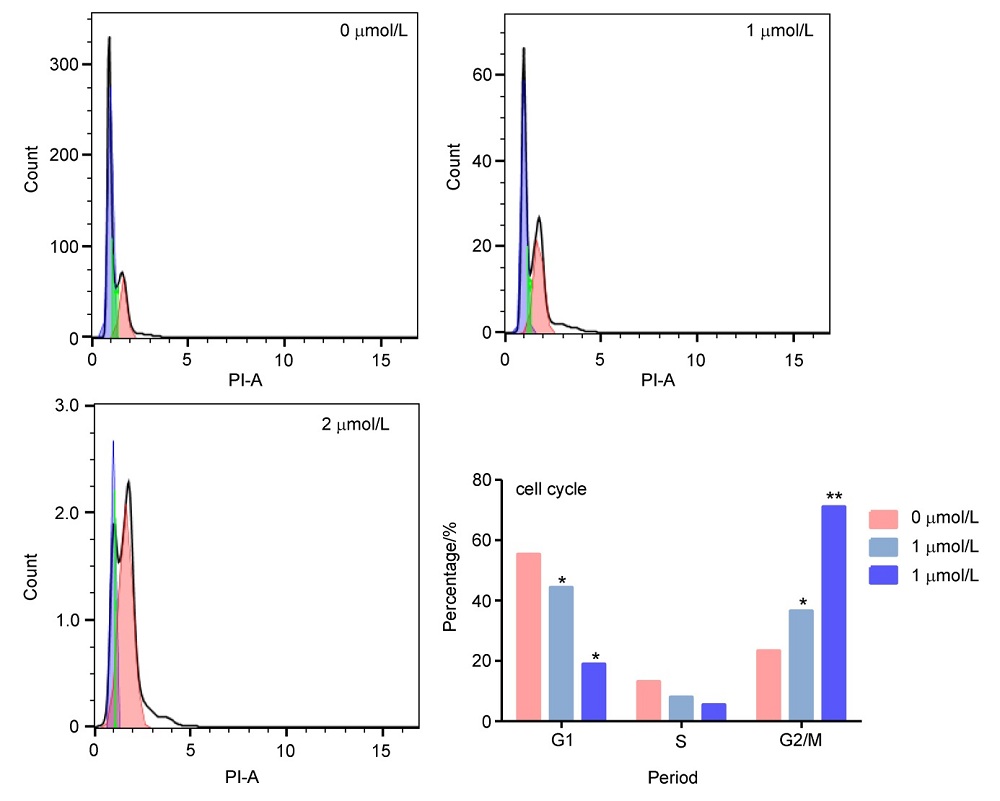
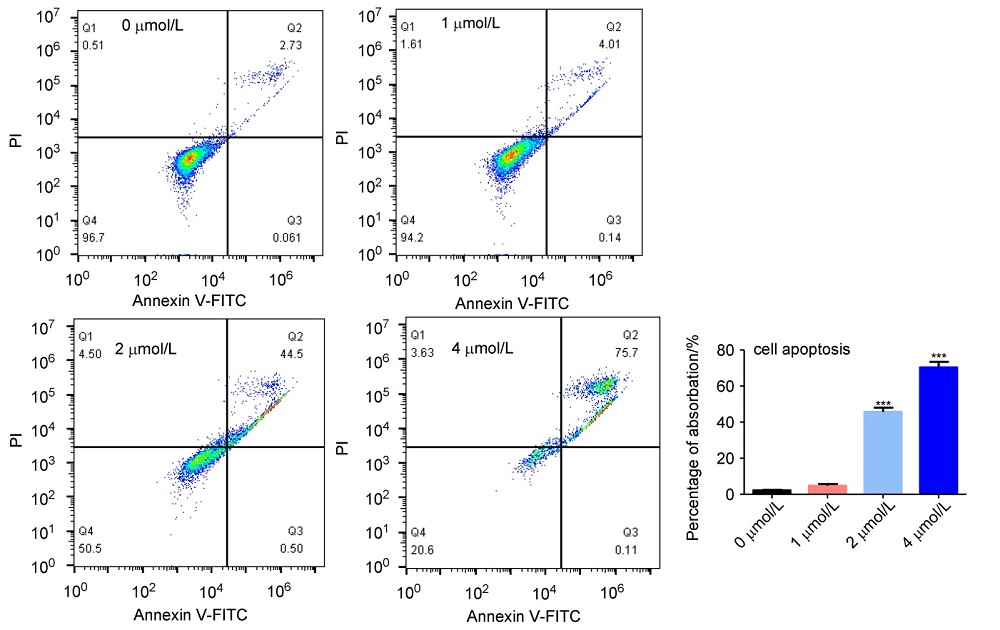
济源冬凌草甲素(JOA)是从河南省济源市收集的冬凌草中纯化得到的二萜类成分, 表现出多种抗肿瘤活性. 为了进一步研究JOA的药用效果, 设计合成了一系列其14-OH苯甲酸衍生物, 随后评估了它们的体外抗增殖活性. 结果证明, 该系列衍生物的抗肿瘤活性优于先导化合物JOA及冬凌草甲素. 其中, 7α,20-内酯-对映-贝壳杉-16-烯-11,15二酮-14β-(2-硝基-5-氯苯甲酸)酯(OJW8-9)的活性最好[对SW1990细胞的IC50=(0.478±0.109) μmol/L]. 进一步的作用机制研究表明, 化合物OJW8-9通过阻断处于G2/M期的SW-1990来抑制细胞增殖, 且具有浓度依赖性, 其可能通过活性氧(ROS)途径诱导细胞凋亡.
王妮, 郑姿君, 贾小苹, 赵梦圆, 王亚蕾, 周臣, 王志佳, 肖泽霖, 刘宏民, 可钰. 济源冬凌草甲素衍生物作为潜在抗肿瘤药物的合成及药理学活性研究[J]. 有机化学, 2023, 43(2): 646-659.
Ni Wang, Zijun Zheng, Xiaoping Jia, Mengyuan Zhao, Yalei Wang, Chen Zhou, Zhijia Wang, Zelin Xiao, Hongmin Liu, Yu Ke. Study on Synthesis and Pharmacological Research of Jiyuan Oridonin A Derivatives as Potential Anti-tumor Drugs[J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 646-659.
| Compd. | R | Yield/% | Compd. | R | Yield/% |
|---|---|---|---|---|---|
| OJW1 | H | 85 | OJW2-1 | 2-OCH3 | 47 |
| OJW2-2 | 3-OCH3 | 75 | OJW2-3 | 4-OCH3 | 63 |
| OJW2-4 | 2,3-(OCH3)2 | 63 | OJW2-5 | 3,4-(OCH3)2 | 41 |
| OJW2-6 | 3,4,5-(OCH3)3 | 82 | OJW3-1 | 2-CH3 | 64 |
| OJW3-2 | 3-CH3 | 67 | OJW3-3 | 4-CH3 | 36 |
| OJW4-1 | 3-Cl | 56 | OJW4-2 | 4-Cl | 74 |
| OJW4-3 | 2,3-Cl2 | 80 | OJW4-4 | 2,6-Cl2 | 78 |
| JW5-1 | 2-Br | 71 | OJW5-2 | 3-Br | 65 |
| OJW5-3 | 4-Br | 67 | OJW6-1 | 2-F | 85 |
| OJW6-2 | 3-F | 82 | OJW6-3 | 4-F | 76 |
| OJW6-4 | 2,3,4-F3 | 76 | OJW6-5 | 2,3,4,5,6-F5 | 54 |
| OJW7-1 | 2-CF3 | 48 | OJW7-2 | 3-CF3 | 52 |
| OJW7-3 | 4-CF3 | 67 | OJW8-1 | 2-NO2 | 85 |
| OJW8-2 | 3-NO2 | 79 | OJW8-3 | 4-NO2 | 71 |
| OJW8-4 | 2,4-(NO2)2 | 64 | OJW8-5 | 3,4-(NO2)2 | 56 |
| OJW8-6 | 3,5-(NO2)2 | 74 | OJW8-7 | 2-NO2-5-OCH3 | 69 |
| OJW8-8 | 2-NO2-5-CH3 | 78 | OJW8-9 | 2-NO2-5-Cl | 70 |
| OJW8-10 | 2-NO2-5-F | 66 | OJW8-11 | 3-NO2-4-F | 54 |
| OJW9-1 | 2-I | 76 | OJW9-2 | 3-I | 82 |
| OJW9-3 | 4-I | 78 | OJW10-1 | 3-CH2Br | 37 |
| OJW10-2 | 4-CH2Br | 27 | OJW11 | 2-CH3-3-F | 37 |
| Compd. | R | Yield/% | Compd. | R | Yield/% |
|---|---|---|---|---|---|
| OJW1 | H | 85 | OJW2-1 | 2-OCH3 | 47 |
| OJW2-2 | 3-OCH3 | 75 | OJW2-3 | 4-OCH3 | 63 |
| OJW2-4 | 2,3-(OCH3)2 | 63 | OJW2-5 | 3,4-(OCH3)2 | 41 |
| OJW2-6 | 3,4,5-(OCH3)3 | 82 | OJW3-1 | 2-CH3 | 64 |
| OJW3-2 | 3-CH3 | 67 | OJW3-3 | 4-CH3 | 36 |
| OJW4-1 | 3-Cl | 56 | OJW4-2 | 4-Cl | 74 |
| OJW4-3 | 2,3-Cl2 | 80 | OJW4-4 | 2,6-Cl2 | 78 |
| JW5-1 | 2-Br | 71 | OJW5-2 | 3-Br | 65 |
| OJW5-3 | 4-Br | 67 | OJW6-1 | 2-F | 85 |
| OJW6-2 | 3-F | 82 | OJW6-3 | 4-F | 76 |
| OJW6-4 | 2,3,4-F3 | 76 | OJW6-5 | 2,3,4,5,6-F5 | 54 |
| OJW7-1 | 2-CF3 | 48 | OJW7-2 | 3-CF3 | 52 |
| OJW7-3 | 4-CF3 | 67 | OJW8-1 | 2-NO2 | 85 |
| OJW8-2 | 3-NO2 | 79 | OJW8-3 | 4-NO2 | 71 |
| OJW8-4 | 2,4-(NO2)2 | 64 | OJW8-5 | 3,4-(NO2)2 | 56 |
| OJW8-6 | 3,5-(NO2)2 | 74 | OJW8-7 | 2-NO2-5-OCH3 | 69 |
| OJW8-8 | 2-NO2-5-CH3 | 78 | OJW8-9 | 2-NO2-5-Cl | 70 |
| OJW8-10 | 2-NO2-5-F | 66 | OJW8-11 | 3-NO2-4-F | 54 |
| OJW9-1 | 2-I | 76 | OJW9-2 | 3-I | 82 |
| OJW9-3 | 4-I | 78 | OJW10-1 | 3-CH2Br | 37 |
| OJW10-2 | 4-CH2Br | 27 | OJW11 | 2-CH3-3-F | 37 |
| Compound | MCF-7 | Ec109 | SW1990 | MGC-803 | Hela |
|---|---|---|---|---|---|
| Oridonin | 8.415±0.440 | 23.359±3.460 | 8.858±0.360 | 12.227±0.211 | 40.863±9.594 |
| JOA | 2.101±0.174 | 4.319±0.688 | 4.188±0.512 | 3.778±0.375 | 6.010±0.191 |
| O-JOA | 1.087±0.179 | 2.269±0.036 | 1.009±0.127 | 1.704±0.074 | 1.757±0.099 |
| OJW1 | 1.608±0.309 | 2.553±0.230 | 0.881±0.071 | 1.753±0.191 | 2.814±0.212 |
| OJW2-1 | 1.745±0.182 | 2.970±0.038 | 1.125±0.133 | 1.634±0.173 | 2.966±0.278 |
| OJW2-2 | 1.969±0.293 | 2.306±0.231 | 0.878±0.139 | 1.652±0.181 | 2.559±0.055 |
| OJW2-3 | 1.862±0.250 | 3.302±0.367 | 1.059±0.170 | 3.122±0.556 | 3.640±0.034 |
| OJW2-4 | 1.731±0.078 | 2.182±0.089 | 1.155±0.219 | 2.152±0.329 | 2.786±0.217 |
| OJW2-5 | 3.114±0.488 | 5.181±0.138 | 0.771±0.120 | 2.421±0.133 | 4.032±0.182 |
| OJW2-6 | 1.479±0.132 | 3.056±0.035 | 1.283±0.163 | 1.023±0.021 | 2.064±0.292 |
| OJW3-1 | 2.742±0.125 | 3.273±0.080 | 1.134±0.106 | 1.222±0.168 | 4.174±0.296 |
| OJW3-2 | 1.996±0.887 | 2.863±0.219 | 1.119±0.047 | 2.123±0.108 | 3.686±0.112 |
| OJW3-3 | 3.195±0.312 | 5.524±0.128 | 2.920±0.398 | 4.109±0.492 | 6.834±0.444 |
| OJW4-1 | 1.708±0.018 | 2.159±0.269 | 1.991±0.047 | 2.063±0.151 | 3.326±0.281 |
| OJW4-2 | 1.976±0.153 | 2.816±0.258 | 2.314±0.909 | 3.463±0.326 | 3.763±0.494 |
| OJW4-3 | 2.119±0.196 | 3.410±0.028 | 1.416±0.202 | 2.891±0.316 | 3.584±0.293 |
| OJW4-4 | 1.619±0.049 | 2.939±0.028 | 1.796±0.249 | 1.513±0.079 | 3.168±0.614 |
| OJW5-1 | 2.648±0.131 | 2.840±0.320 | 1.574±0.051 | 2.207±0.403 | 3.072±0.110 |
| OJW5-2 | 1.660±0.231 | 3.133±0.176 | 1.114±0.163 | 3.133±0.617 | 3.182±0.103 |
| OJW5-3 | 2.175±0.444 | 6.521±1.220 | 1.694±0.023 | 3.276±0.059 | 3.930±0.247 |
| OJW6-2 | 1.661±0.046 | 2.447±0.061 | 1.314±0.279 | 3.573±0.316 | 3.664±0.352 |
| OJW6-3 | 2.830±0.085 | 3.902±0.258 | 1.227±0.102 | 2.235±0.076 | 5.184±0.075 |
| OJW6-4 | 1.604±0.063 | 1.902±0.125 | 0.969±0.048 | 2.416±0.216 | 3.314±0.289 |
| OJW6-5 | 1.160±0.113 | 2.061±0.234 | 1.098±0.176 | 1.045±0.09 | 2.062±0.059 |
| OJW7-1 | 2.413±0.065 | 4.268±0.174 | 1.687±0.181 | 3.407±0.689 | 6.863±0.297 |
| OJW7-2 | 2.796±0.106 | 5.067±0.502 | 1.142±0.071 | 1.926±0.023 | 4.269±0.487 |
| OJW7-3 | 1.839±0.145 | 2.556±0.110 | 1.601±0.101 | 2.258±0.310 | 4.085±0.180 |
| OJW8-1 | 1.255±0.157 | 2.692±0.257 | 0.905±0.113 | 1.771±0.058 | 3.028±0.098 |
| OJW8-2 | 1.183±0.071 | 3.153±0.255 | 1.348±0.212 | 1.886±0.300 | 3.097±0.424 |
| OJW8-3 | 1.283±0.050 | 2.486±0.329 | 1.088±0.129 | 2.220±0.363 | 3.698±0.487 |
| OJW8-4 | 1.013±0.038 | 2.967±0.017 | 0.708±0.073 | 2.350±0.025 | 2.767±0.112 |
| OJW8-5 | 2.468±0.326 | 5.283±0.164 | 1.765±0.169 | 4.476±0.287 | 6.688±0.771 |
| OJW8-6 | 0.667±0.081 | 2.653±0.208 | 0.938±0.005 | 1.471±0.318 | 3.265±0.290 |
| OJW8-7 | 1.281±0.034 | 1.974±0.219 | 1.669±0.042 | 2.174±0.215 | 6.629±0.715 |
| OJW8-8 | 1.701±0.124 | 2.501±0.174 | 1.871±0.040 | 2.265±0.066 | 7.854±0.098 |
| OJW8-9 | 0.804±0.013 | 1.452±0.126 | 0.478±0.109 | 1.097±0.125 | 1.864±0.073 |
| OJW8-10 | 2.309±0.056 | 2.777±0.280 | 1.557±0.223 | 2.196±0.263 | 9.159±1.309 |
| OJW8-11 | 1.263±0.140 | 1.523±0.251 | 0.858±0.119 | 2.040±0.130 | 3.729±0.090 |
| OJW9-1 | 2.101±0.131 | 5.364±0.416 | 1.130±0.166 | 1.887±0.135 | 4.322±0.323 |
| OJW9-2 | 2.449±0.135 | 3.578±0.362 | 1.989±0.016 | 1.648±0.243 | 5.210±0.271 |
| OJW9-3 | 1.957±0.022 | 3.444±0.101 | 1.152±0.197 | 2.187±0.143 | 3.664±0.425 |
| OJW10-1 | 0.886±0.015 | 1.746±0.107 | 0.726±0.053 | 0.963±0.179 | 0.970±0.055 |
| OJW10-2 | 1.506±0.179 | 3.137±0.191 | 0.936±0.019 | 1.908±0.225 | 5.179±0.509 |
| OJW11 | 1.267±0.074 | 3.568±0.365 | 0.974±0.064 | 1.983±0.260 | 3.135±0.251 |
| Compound | MCF-7 | Ec109 | SW1990 | MGC-803 | Hela |
|---|---|---|---|---|---|
| Oridonin | 8.415±0.440 | 23.359±3.460 | 8.858±0.360 | 12.227±0.211 | 40.863±9.594 |
| JOA | 2.101±0.174 | 4.319±0.688 | 4.188±0.512 | 3.778±0.375 | 6.010±0.191 |
| O-JOA | 1.087±0.179 | 2.269±0.036 | 1.009±0.127 | 1.704±0.074 | 1.757±0.099 |
| OJW1 | 1.608±0.309 | 2.553±0.230 | 0.881±0.071 | 1.753±0.191 | 2.814±0.212 |
| OJW2-1 | 1.745±0.182 | 2.970±0.038 | 1.125±0.133 | 1.634±0.173 | 2.966±0.278 |
| OJW2-2 | 1.969±0.293 | 2.306±0.231 | 0.878±0.139 | 1.652±0.181 | 2.559±0.055 |
| OJW2-3 | 1.862±0.250 | 3.302±0.367 | 1.059±0.170 | 3.122±0.556 | 3.640±0.034 |
| OJW2-4 | 1.731±0.078 | 2.182±0.089 | 1.155±0.219 | 2.152±0.329 | 2.786±0.217 |
| OJW2-5 | 3.114±0.488 | 5.181±0.138 | 0.771±0.120 | 2.421±0.133 | 4.032±0.182 |
| OJW2-6 | 1.479±0.132 | 3.056±0.035 | 1.283±0.163 | 1.023±0.021 | 2.064±0.292 |
| OJW3-1 | 2.742±0.125 | 3.273±0.080 | 1.134±0.106 | 1.222±0.168 | 4.174±0.296 |
| OJW3-2 | 1.996±0.887 | 2.863±0.219 | 1.119±0.047 | 2.123±0.108 | 3.686±0.112 |
| OJW3-3 | 3.195±0.312 | 5.524±0.128 | 2.920±0.398 | 4.109±0.492 | 6.834±0.444 |
| OJW4-1 | 1.708±0.018 | 2.159±0.269 | 1.991±0.047 | 2.063±0.151 | 3.326±0.281 |
| OJW4-2 | 1.976±0.153 | 2.816±0.258 | 2.314±0.909 | 3.463±0.326 | 3.763±0.494 |
| OJW4-3 | 2.119±0.196 | 3.410±0.028 | 1.416±0.202 | 2.891±0.316 | 3.584±0.293 |
| OJW4-4 | 1.619±0.049 | 2.939±0.028 | 1.796±0.249 | 1.513±0.079 | 3.168±0.614 |
| OJW5-1 | 2.648±0.131 | 2.840±0.320 | 1.574±0.051 | 2.207±0.403 | 3.072±0.110 |
| OJW5-2 | 1.660±0.231 | 3.133±0.176 | 1.114±0.163 | 3.133±0.617 | 3.182±0.103 |
| OJW5-3 | 2.175±0.444 | 6.521±1.220 | 1.694±0.023 | 3.276±0.059 | 3.930±0.247 |
| OJW6-2 | 1.661±0.046 | 2.447±0.061 | 1.314±0.279 | 3.573±0.316 | 3.664±0.352 |
| OJW6-3 | 2.830±0.085 | 3.902±0.258 | 1.227±0.102 | 2.235±0.076 | 5.184±0.075 |
| OJW6-4 | 1.604±0.063 | 1.902±0.125 | 0.969±0.048 | 2.416±0.216 | 3.314±0.289 |
| OJW6-5 | 1.160±0.113 | 2.061±0.234 | 1.098±0.176 | 1.045±0.09 | 2.062±0.059 |
| OJW7-1 | 2.413±0.065 | 4.268±0.174 | 1.687±0.181 | 3.407±0.689 | 6.863±0.297 |
| OJW7-2 | 2.796±0.106 | 5.067±0.502 | 1.142±0.071 | 1.926±0.023 | 4.269±0.487 |
| OJW7-3 | 1.839±0.145 | 2.556±0.110 | 1.601±0.101 | 2.258±0.310 | 4.085±0.180 |
| OJW8-1 | 1.255±0.157 | 2.692±0.257 | 0.905±0.113 | 1.771±0.058 | 3.028±0.098 |
| OJW8-2 | 1.183±0.071 | 3.153±0.255 | 1.348±0.212 | 1.886±0.300 | 3.097±0.424 |
| OJW8-3 | 1.283±0.050 | 2.486±0.329 | 1.088±0.129 | 2.220±0.363 | 3.698±0.487 |
| OJW8-4 | 1.013±0.038 | 2.967±0.017 | 0.708±0.073 | 2.350±0.025 | 2.767±0.112 |
| OJW8-5 | 2.468±0.326 | 5.283±0.164 | 1.765±0.169 | 4.476±0.287 | 6.688±0.771 |
| OJW8-6 | 0.667±0.081 | 2.653±0.208 | 0.938±0.005 | 1.471±0.318 | 3.265±0.290 |
| OJW8-7 | 1.281±0.034 | 1.974±0.219 | 1.669±0.042 | 2.174±0.215 | 6.629±0.715 |
| OJW8-8 | 1.701±0.124 | 2.501±0.174 | 1.871±0.040 | 2.265±0.066 | 7.854±0.098 |
| OJW8-9 | 0.804±0.013 | 1.452±0.126 | 0.478±0.109 | 1.097±0.125 | 1.864±0.073 |
| OJW8-10 | 2.309±0.056 | 2.777±0.280 | 1.557±0.223 | 2.196±0.263 | 9.159±1.309 |
| OJW8-11 | 1.263±0.140 | 1.523±0.251 | 0.858±0.119 | 2.040±0.130 | 3.729±0.090 |
| OJW9-1 | 2.101±0.131 | 5.364±0.416 | 1.130±0.166 | 1.887±0.135 | 4.322±0.323 |
| OJW9-2 | 2.449±0.135 | 3.578±0.362 | 1.989±0.016 | 1.648±0.243 | 5.210±0.271 |
| OJW9-3 | 1.957±0.022 | 3.444±0.101 | 1.152±0.197 | 2.187±0.143 | 3.664±0.425 |
| OJW10-1 | 0.886±0.015 | 1.746±0.107 | 0.726±0.053 | 0.963±0.179 | 0.970±0.055 |
| OJW10-2 | 1.506±0.179 | 3.137±0.191 | 0.936±0.019 | 1.908±0.225 | 5.179±0.509 |
| OJW11 | 1.267±0.074 | 3.568±0.365 | 0.974±0.064 | 1.983±0.260 | 3.135±0.251 |



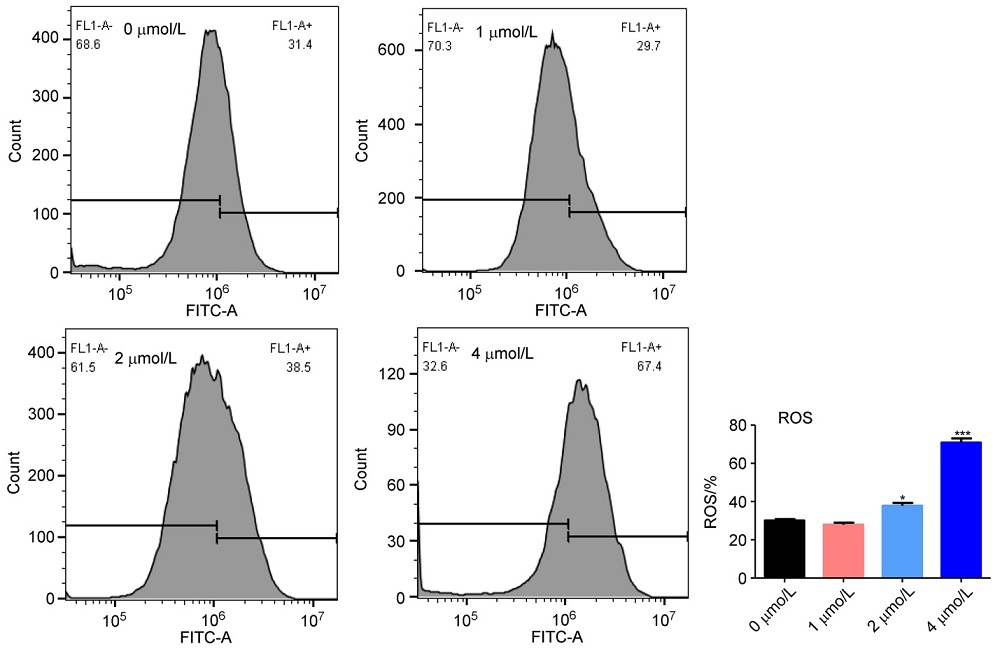

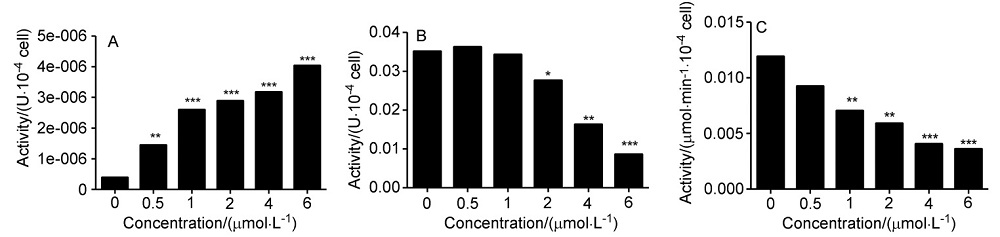
| [1] |
Sun, H. D.; Huang, S. X.; Han, Q. B. Nat. Prod. Rep. 2006, 23, 673.
pmid: 17003905 |
| [2] |
Liu, X.; Yang, J.; Wang, W. G.; Li, Y.; Wu, J. Z.; Pu, J. X.; Sun, H. D. J. Nat. Prod. 2015, 78, 196.
doi: 10.1021/np5006136 pmid: 25590529 |
| [3] |
Yuan, K.; Wu, C. Z.; Zhang, X. Y.; Sun, W.; Zheng, Y. Chin. J. Mod. Appl. Pharm. 2004, 21, 213. (in Chinese)
|
|
(袁珂, 吴崇珍, 张晓明, 孙伟, 郑昱, 中国现代应用药学杂志, 2004, 21, 213.)
|
|
| [4] |
Fujita, E.; Nagao, Y.; Kaneko, K.; Nakazawa, S.; Kuroda, H. Chem. Pharm. Bull. 1976, 24, 2118.
doi: 10.1248/cpb.24.2118 |
| [5] |
Fujita, E.; Nagao, Y.; Kohno, T.; Matsuda, M.; Ozaki, M. Chem. Pharm. Bull. 1981, 29, 3208.
doi: 10.1248/cpb.29.3208 |
| [6] |
Luo, X.; Pu, J.-X.; Xiao, W.-L.; Zhao, Y.; Gao, X.-M.; Li, X.-N.; Zhang, H.-B.; Wang, Y.-Y.; Li, Y.; Sun, H.-D. J. Nat. Prod. 2010, 73, 1112.
doi: 10.1021/np100110u pmid: 20521771 |
| [7] |
Han, Q. B.; Zhang, J. X.; Lu, Y.; Wu, Y. S.; Zheng, Q. T.; Sun, H. D. Planta Med. 2004, 70, 581.
doi: 10.1055/s-2004-827165 |
| [8] |
Han, Q. B.; Jiang, B.; Zhang, J. X.; Niu, X. M.; Sun, H. D. Helv. Chim. Acta 2003, 86, 773.
doi: 10.1002/hlca.200390077 |
| [9] |
Cheng, Y.; Qiu, F.; Ye, Y. C.; Tashiro, S.; Onodera, S.; Ikejima, T. Arch. Biochem. Biophys. 2009, 490, 70.
doi: 10.1016/j.abb.2009.08.011 pmid: 19699177 |
| [10] |
Bao, R.; Shu, Y.; Wu, X.; Weng, H.; Ding, Q.; Cao, Y.; Li, M.; Mu, J.; Wu, W.; Ding, Q.; Tan, Z.; Liu, T.; Jiang, L.; Hu, Y.; Gu, J.; Liu, Y. BMC Cancer 2014, 14, 217.
doi: 10.1186/1471-2407-14-217 |
| [11] |
Cheng, Y.; Qiu, F.; Ikejima, T. Autophagy 2009, 5, 430.
pmid: 19202353 |
| [12] |
Xia, S.; Zhang, X.; Li, C.; Guan, H. Saudi Pharm. J. 2017, 25, 638.
doi: 10.1016/j.jsps.2017.04.037 |
| [13] |
Xu, J.; Yang, J.; Ran, Q.; Wang, L.; Liu, J.; Wang, Z.; Wu, X.; Hua, W.; Yuan, S.; Zhang, L.; Shen, M.; Ding, Y. Bioorg. Med. Chem. Lett. 2008, 18, 4741.
doi: 10.1016/j.bmcl.2008.06.097 |
| [14] |
Wang, L.; Li, D.; Xu, S.; Cai, H.; Yao, H.; Zhang, Y.; Jiang, J.; Xu, J. Eur. J. Med. Chem. 2012, 52, 242.
|
| [15] |
Li, D.; Han, T.; Tian, K.; Tang, S.; Xu, S.; Hu, X.; Wang, L.; Li, Z.; Hua, H.; Xu, J. Bioorg. Med. Chem. Lett. 2016, 26, 4191.
|
| [16] |
Xu, S.; Yao, H.; Luo, S.; Zhang, Y. K.; Yang, D. H.; Li, D.; Wang, G.; Hu, M.; Qiu, Y.; Wu, X.; Yao, H.; Xie, W.; Chen, Z. S.; Xu, J. A J. Med. Chem. 2017, 60, 1449.
doi: 10.1021/acs.jmedchem.6b01652 |
| [17] |
Luo, D.-D.; Peng, K.; Yang, J.-Y.; Piyachaturawat, P.; Saengsawang, W.; Ao, L.; Zhao, W.-Z.; Tang, Y.; Wan, S.-B. RSC Adv. 2018, 8, 29548.
doi: 10.1039/C8RA05728A |
| [18] |
Luo, D.; Yi, Y.; Peng, K.; Liu, T.; Yang, J.; Liu, S.; Zhao, W.; Qu, X.; Yu, W.; Gu, Y.; Wan, S. Eur. J. Med. Chem. 2019, 178, 365.
doi: 10.1016/j.ejmech.2019.06.006 |
| [19] |
Ke, Y.; Wang, W.; Zhao, L. F.; Liang, J. J.; Liu, Y.; Zhang, X.; Feng, K.; Liu, H. M. Bioorg. Med. Chem. Lett. 2018, 26, 4761.
doi: 10.1016/j.bmc.2017.11.005 |
| [20] |
Ke, Y.; Liang, J. J.; Hou, R. J.; Li, M. M.; Zhao, L. F.; Wang, W.; Liu, Y.; Xie, H.; Yang, R. H.; Hu, T. X.; Wang, J. Y.; Liu, H. M. Eur. J. Med. Chem. 2018, 157, 1249.
doi: 10.1016/j.ejmech.2018.08.056 |
| [21] |
Xu, S.; Yao, H.; Hu, M.; Li, D.; Zhu, Z.; Xie, W.; Yao, H.; Wu, L.; Chen, Z.S.; Xu, J. J. Nat. Prod. 2017, 80, 2391.
doi: 10.1021/acs.jnatprod.7b00057 |
| [22] |
Yao, H.; Xie, S.; Ma, X.; Liu, J.; Wu, H.; Lin, A.; Yao, H.; Li, D.; Xu, S.; Yang, D.H.; Chen, Z. S.; Xu, J. J. Med. Chem. 2020, 63, 8157.
doi: 10.1021/acs.jmedchem.0c00408 |
| [23] |
Ke, Y.; Hu, T. X.; Huo, J. F.; Yan, J. K.; Wang, J. Y.; Yang, R. H.; Xie, H.; Liu, Y.; Wang, N.; Zheng, Z. J.; Sun, Y. X.; Wang, C.; Du, J.; Liu, H. M. Eur. J. Med. Chem. 2019, 182, 111645.
doi: 10.1016/j.ejmech.2019.111645 |
| [24] |
Huo, J. F.; Hu, T. X.; Dong, Y. L.; Zhao, J. Z.; Liu, X. J.; Li, L. L.; Zhang, X. Y.; Li, Y. F.; Liu, H. M.; Ke, Y.; Wang, C. Eur. J. Med. Chem. 2020, 208, 112789.
doi: 10.1016/j.ejmech.2020.112789 |
| [25] |
Yan, X.-B. Ph.D. Dissertation, Zhengzhou University, Zhengzhou, 2002. (in Chinese)
|
|
(闫学斌, 博士论文, 郑州大学, 郑州, 2002.)
|
| [1] | 罗云, 高谕康, 燕鹏程, 朱伟明. 浅蓝霉素H的发酵优化与浅蓝苷K的化学合成[J]. 有机化学, 2022, 42(9): 2840-2849. |
| [2] | 高潮, 司晓杰, 池玲玲, 王浩, 戴洪林, 刘丽敏, 汪正捷, 张洋, 王涛, 周耀传, 郑甲信, 可钰, 刘宏民, 张秋荣. 含苯甲醚结构的2,4,5,6-四取代嘧啶衍生物的合成及抗增殖活性研究[J]. 有机化学, 2022, 42(6): 1677-1686. |
| [3] | 高潮, 张玉桐, 池玲玲, 王浩, 马家婕, 毕梦鑫, 戴洪林, 司晓杰, 刘丽敏, 张洋, 郑甲信, 可钰, 刘宏民, 张秋荣. 新型2,4,6-三取代嘧啶衍生物的合成及抗增殖活性评价[J]. 有机化学, 2022, 42(11): 3824-3834. |
| [4] | 张涛, 卫海沅, 马雯, 李张媛, 胡盼盼, 周楠茜, 贺建超, 李婷, 苏明明, 白素平. 新型蓝萼甲素-1,2,3-三氮唑类衍生物的合成与抗增殖活性研究[J]. 有机化学, 2022, 42(11): 3668-3683. |
| [5] | 张燕, 王芸芸, 赵雨珣, 张成龙, 谷文, 王忠龙, 朱永强, 王石发. 樟脑基缩氨基硫脲衍生物通过活性氧(ROS)介导的线粒体途径诱导人乳腺癌细胞的G2期阻滞和凋亡[J]. 有机化学, 2020, 40(8): 2374-2386. |
| [6] | 张路野, 张洋, 包崇男, 杨鹏, 李二冬, 孟娅琪, 崔飞, 周蕊, 黄诗雨, 郑甲信, 单丽红, 刘宏民, 张秋荣. 新型1,3-二取代酞嗪酮类衍生物的合成及抗肿瘤活性研究[J]. 有机化学, 2020, 40(3): 794-800. |
| [7] | 矫春鹏, 刘媛媛, 路文娟, 张平平, 王延风. 检测活性氮/活性氧的分子荧光探针[J]. 有机化学, 2019, 39(3): 591-616. |
| [8] | 孙海燕, 孙宏顺, 刘明珍, 黄伟, 杨光富. 基于二硫代氨基甲酸酯活性亚结构的先导优化及其抗增殖活性[J]. 有机化学, 2018, 38(8): 2067-2075. |
| [9] | 后际挺, 李坤, 覃彩芹, 余孝其. 活性氧簇的小分子荧光探针研究进展[J]. 有机化学, 2018, 38(3): 612-628. |
| [10] | 张娅玲, 马沙沙, 李夏冰, 侯巧丽, 吕梦娇, 郝云霞, 王伟, 李宝林. 吡咯并三嗪衍生物的合成及其对肿瘤细胞增殖的抑制活性[J]. 有机化学, 2018, 38(12): 3270-3277. |
| [11] | 王延宝, 赵宝祥. 次氯酸荧光探针的研究进展[J]. 有机化学, 2016, 36(7): 1539-1554. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||