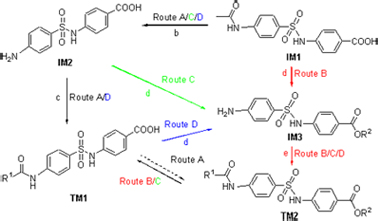
| [1] Xu, Y. P.; Etgen, G. J.; Broderick, C. L.; Canada, E.; Gonzalez, I.; Lamar, J.; Montrose-Rafizadeh, C.; Oldham, B. A.; Osborne, J. J., Xie, C. Y.; Shi, Q.; Winneroski, L. L.; York, J.; Yumibe, N.; Zink, R.; Mantlo, N. J. Med. Chem. 2006, 49, 5649. [2] Yoshida, K.; Hishida, A.; Iida, O.; Hosokawa, K.; Kawabata, J. J Agric. Food Chem. 2008, 56, 4367. [3] Mohler, M. L.; He Y. L.; Wu Z. Z.; Hwang, D. J.; Miller, D. D. Med. Res. Rev. 2009, 29, 125. [4] Kim, S. J.; Jung, M. H.; Yoo, K. H.; Cho, J. H.; Oh, C. H. Bioorg. Med. Chem. Lett. 2008, 18, 5815. [5] Temperini, C.; Cecchi, A.; Scozzafava, A.; Supuran, C. T. Bioorg. Med. Chem. Lett. 2008, 18, 2567. [6] Rothschild, C. M.; Hines, M. T.; Breuhaus, B.; Gay, J.; Sellon, D. C. J. Vet. Intern. Med. 2004, 18, 370. [7] Tanaka, H.; Ohshima, N.; Hidaka, H. Mol. Pharmacol. 1999, 55, 356. [8] Yang, H. T.; Endoh, M. Eur. J. Pharmacol. 1996, 312, 281. [9] Hao, G. F.; Yang, G. F. Chin. J. Org. Chem. 2008, 28, 1545 (in Chinese).(郝格非, 杨光富, 有机化学, 2008, 28, 1545.) [10] Seri, K.; Kazuko, S.; Katsumi, K.; Yorishige, I.; Hiroyuki, A. Eur. J. Pharmacol. 2000, 389, 253. [11] Basset, G. J. C.; Quinlivan, E. P.; Ravanel, S.; Rébeillé, F.; Nichols, B. P.; Shinozaki, K.; Seki, M.; Adams-Phillips, L. C.; Giovannoni, J. J.; Gregory III, J. F.; Hanson, A. D. Proc. Natl. Acad. Sci. U. S. A. 2004, 101, 1496. [12] Pawelczak, K.; Jones, T. R.; Kempny, M.; Jackman, A. L.; Newell, D. R.; Krzyzanowski, L.; Rzeszotarska, B. J. Med. Chem. 1989, 32, 160. [13] Lahue, B. R.; Wan, Z. K.; Snyder, J. K. J. Org. Chem. 2003, 68, 4345. [14] Ohkanda, J.; Buckner, F. S.; Lockman, J. W.; Yokoyama, K.; Carrico, D.; Eastman, R.; Luca-Fradley, K. D.; Davies, W.; Croft, S. L.; Voorhis, W. C. V.; Gelb, M. H.; Sebti, S. M.; Hamilton, A. D. J. Med. Chem. 2004, 47, 432. [15] Sun, J.; Yang, Y. S.; Li, W.; Zhang, Y. B.; Wang, X. L.; Tang, J. F.; Zhu, H. L. Bioorg. Med. Chem. Lett. 2011, 21, 6116. [16] Prabhu, P. P.; Shastry, C. S.; Pande, S.; Pai, A. J. Pharm. Res. 2011, 4, 2209. [17] Qiu, J. Y.; Xu, B.; Huang, Z. M.; Pan, W. D.; Cao, P. X.; Liu, C. X.; Hao, X. J.; Song, B. A.; Liang, G. Y. Bioorg. Med. Chem. 2011, 19, 5352. [18] Chen, X. F.; Wu, Y. B.; Jin, J.; Wang, R. Z.; Wang, C.; Liu, J. Acta Pharm. Sin. 2010, 45, 263 (in Chinese).(陈晓芳, 武燕彬, 金洁, 王瑞贞, 王翀, 刘浚, 药学学报, 2010, 45, 263.) [19] Mulongo, G.; Mbabazi, J.; Odongkara, B.; Twinomuhwezi, H.; Mpango, G. B. Res. J. Chem. Sci. 2011, 1, 102. [20] Pfefferkorn, J. A.; Song, Y. T.; Sun, K. L.; Miller, S. R.; Trivedi, B. K.; Choi, C.; Sorenson, R. J.; Bratton, L. D.; Unangst, P. C.; Larsen, S. D.; Poel, T. J.; Cheng, X. M.; Lee, C.; Erasga, N.; Auerbach, B.; Askew, V.; Dillon, L.; Hanselman, J. C.; Lin, Z. W.; Lu, G.; Robertson, A.; Olsen, K.; Mertz, T.; Sekerke, C.; Pavlovsky, A.; Harris, M. S.; Bainbridge, G.; Caspers, N.; Chen, H. F.; Eberstadt, M. Bioorg. Med. Chem. Lett. 2007, 17, 4538. [21] Dhananjeyan, M. R.; Trendel, J. A.; Bykowski, C.; Sarver, J. G.; Ando, H.; Erhardt, P. W. J. Chromatogr., B 2008, 867, 247. [22] Yang, D. C.; Yan, J. F.; Xu, J.; Ye, F.; Zhou, Z. W.; Zhang, W. Y.; Fan, L.; Chen, X. Acta Pharm. Sin. 2010, 45, 66 (in Chinese).(杨大成, 晏菊芳, 许荩, 叶飞, 周祖文, 张蔚瑜, 范莉, 陈欣, 药学学报, 2010, 45, 66.) [23] Zhang, K.; Yan, J. F.; Tang, X. M.; Liu, H. P.; Fan, L.; Zhou, G. M.; Yang, D. C. Acta Pharm. Sin. 2011, 46, 412 (in Chinese).(张坤, 晏菊芳, 唐雪梅, 刘红萍, 范莉, 周光明, 杨大成, 药学学报, 2011, 46, 412.) [24] Tang, X. M.; Yan, J. F.; Zhang, Y. X.; Zhang, W. Y.; Su, X. Y.; Chen, X.; Zhou, Z. W.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 1790 (in Chinese).(唐雪梅, 晏菊芳, 张映霞, 张蔚瑜, 苏小燕, 陈欣, 周祖文, 杨大成, 有机化学, 2009, 29, 1790.) [25] Song, X. L.; Yan, J. F.; Fan, L.; Chen, X.; Xu, J.; Zhou, Z. W.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 606 (in Chinese).(宋小礼, 晏菊芳, 范莉, 陈欣, 许荩, 周祖文, 杨大成, 有机化学, 2009, 29, 606.) [26] Tang, X. M.; Fan, L.; Yu, H. X.; Liao, Y. H.; Yang, D. C. Chin. J. Org. Chem. 2009, 29, 595 (in Chinese).(唐雪梅, 范莉, 于红霞, 廖玉华, 杨大成, 有机化学, 2009, 29, 595.) [27] Novacek, A.; Sedlackova, V.; Korner, J.; Danek, J. CS 266792, 1990 [Chem. Abstr. 1991, 114, 246957]. [28] Rajagopalan, S. Proc.-Indian Acad. Sci., Sect. A 1943, 18A, 108. [29] Yang, D. C.; Wang, L. F.; Fan, L.; Wang, G. B.; Shang-Guan, R. Y.; Sun, J.; Li, C. Z.; Yang, L. CN 102153481, 2011 [Chem. Abstr. 2011, 155, 352231]. [30] Wang, G. B.; Wang, L. F.; Li, C. Z.; Sun, J.; Zhou, G. M.; Yang, D. C. Res. Chem. Intermed. 2012, 38, 77. [31] Meshram, G. A.; Patil, V. D. Tetrahedron Lett. 2009, 50, 1117. [32] Bahrami, K.; Khodaei, M. M.; Soheilizad, M. J. Org. Chem. 2009, 74, 9287. [33] Palakurthy, N. B.; Mandal, B. Tetrahedron Lett. 2011, 52, 7132. [34] Yu, X. F.; Lu, X. Y. Adv. Synth. Catal. 2011, 353, 2805. [35] Luo, X. X.; Bai, H.; Xue, X. Y.; Hou, Z.; Zhou, Y.; Sang, G. J.; Meng, J. R. CN 101857562, 2010 [Chem. Abstr. 2010, 153, 554743]. [36] Pastor-Navarro, N.; García-Bover, C.; Maquieira, á.; Puchades, R. Anal. Bioanal. Chem. 2004, 379, 1088. [37] Mirza, S. M.; Mustafa, G.; Khan, I. U.; Zia-Ur-Rehman, M.; Shafiq, M. Acta Crystallogr., Sect. E 2010, E67, o25. [38] Chitranshi, P.; Xue, L. Bioorg. Med. Chem. Lett. 2011, 21, 6357. [39] Motoshima, K.; Ishikawa, M.; Hashimoto, Y.; Sugita, K. Bioorg. Med. Chem. 2011, 19, 3156. [40] Ismail, N. S. M.; Hattori, M. Bioorg. Med. Chem. 2011, 19, 374. [41] Hutchinson, J. H.; Li, Y. W.; Arruda, J. M.; Baccei, C.; Bain, G.; Chapman, C.; Correa, L.; Darlington, J.; King, C. D.; Lee, C.; Lorrain, D.; Prodanovich, P.; Rong, H. J.; Santini, A.; Stock, N.; Prasit, P.; Evans, J. F. J. Med. Chem. 2009, 52, 5803. [42] Suryadevara, P. K.; Olepu, S.; Lockman, J. W.; Ohkanda, J.; Karimi, M.; Verlinde, C. L. M. J.; Kraus, J. M.; Schoepe, J.; Voorhis, W. C. V.; Hamilton, A. D.; Buckner, F. S.; Gelb, M. H. J. Med. Chem. 2009, 52, 3703. [43] Takaya, J.; Miyashita, Y.; Kusama, H.; Iwasawa, N. Tetrahedron 2011, 67, 4455. [44] Rewcastle, G. W.; Gamage, S. A.; Flanagan, J. U.; Frederick, R.; Denny, W. A.; Baguley, B. C.; Kestell, P.; Singh, R.; Kendall, J. D.; Marshall, E. S.; Lill, C. L.; Lee, W. J.; Kolekar, S.; Buchanan, C. M.; Jamieson, S. M. F.; Shepherd, P. R. J. Med. Chem. 2011, 54, 7105. [45] Brenna, E.; Fuganti, C.; Gatti, F. G.; Parmeggiani, F. Tetrahedron: Asymmetry 2009, 20, 2594. [46] Sliman, F.; Desmaele, D. Synthesis 2010, 619. [47] Shi, G. Q.; Dropinski, J. F.; McKeever, B. M.; Xu, S. H.; Becker, J. W.; Berger, J. P.; MacNaul, K. L.; Elbrecht, A.; Zhou, G. C.; Doebber, T. W.; Wang, P. R.; Chao, Y. S.; Forrest, M.; Heck, J. V.; Moller, D. E.; Jones, A. B. J. Med. Chem. 2005, 48, 4457. [48] Kumar, A.; Maurya, R. A.; Sharma, S.; Ahmad, P.; Singh, A. B.; Tamrakar, A. K.; Srivastava, A. K. Bioorg. Med. Chem. 2009, 17, 5285. [49] Yang, D. C.; Yan, J. F.; Wang, L. F.; Ye, F.; Fan, L.; Mou, X.; Li, T. J.; Liu, H. L.; Chen, W. D.; Xu, J.; Song, X. L. CN 101735286, 2010 [Chem. Abstr. 2010, 153, 116535]. [50] Corradi, V.; Mancini, M.; Santucci, M. A.; Carlomagno, T.; Sanfelice, D.; Mori, M.; Vignaroli, G.; Falchi, F.; Manetti, F.; Radi, M.; Botta, M. Bioorg. Med. Chem. Lett. 2011, 21, 6867. [51] Yao, Z. Y.; Yang, D. C.; Fan, L. Chin. J. Med. Chem. 2003, 13, 16 (in Chinese).(姚志勇, 杨大成, 范莉, 中国药物化学杂志, 2003, 13, 16.) [52] Gupta, P.; Paul, S. Green Chem. 2011, 13, 2365. [53] Rajput, A. P.; Gore, R. P. Der Pharma Chem. 2011, 3, 409. [54] Balaskar, R. S.; Gavade, S. N.; Mane, M. S.; Shingare, M. S.; Mane, D. V. Green Chem. Lett. Rev. 2011, 4, 91. [55] Chou, C. T.; Yellol, G. S.; Chang, W. J.; Sun, M. L.; Sun, C. M. Tetrahedron 2011, 67, 2110. [56] Berger, J.; Moller, D. E. Annu. Rev. Med. 2002, 53, 409. Krentz, A. J.; Bailey, C. J. Drugs 2005, 65, 385. |