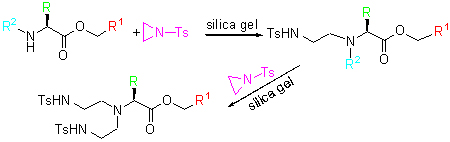
| [1] Chandrasekhar, S.; Narsihmulu, C.; Shameem Sultana, S. Tetrahedron Lett. 2002, 43, 7361 and references cited therein.[2] (a) Ikariya, T.; Blacker, A. J. Acc. Chem. Res. 2007, 40, 1300.(b) Palmer, M. J.; Wills, M. Tetrahedron: Asymmetry 1999, 10, 2045.(c) Zhang, B.; Wang, H.; Lin, G. Q.; Xu, M. H. Eur. J. Org. Chem. 2011, 4205.(d) Jiang, Y. T.; Jiang, Q. Z.; Zhang, X. M. J. Am. Chem. Soc. 1998, 120, 3817.(e) Geoghegan, P.; O'Leary, P. Tetrahedron: Asymmetry 2010, 21, 867.[3] Scheuermann, J. E. W.; Ilyashenko, G.; Griffiths, D. V.; Watkinson, M. Tetrahedron: Asymmetry 2002, 13, 269.[4] (a) Bisai, A.; Prasad, B. A. B.; Singh, V. K. Tetrahedron Lett. 2005, 46, 7935.(b) Thierry, J.; ServaJean, V. Tetrahedron Lett. 2004, 45, 821.(c) Parrodi, C. A.; Vázquez, V.; Quintero, L.; Juaristi, E. Synth. Commun. 2001, 31, 3295.(d) Yadav, J. S.; Reddy, B. V. S.; Jyothirmai, B.; Murty, M. S. R. Synlett 2002, 53.[5] Sekar, G.; Singh, V. K. J. Org. Chem. 1999, 64, 2537.[6] Rinaudo, G.; Narizuka, S.; Askari, N.; Crousse, B.; Bonnet-Delpon, D. Tetrahedron Lett. 2006, 47, 2065.[7] Ye, W.; Leow, D.; Goh, S. L. M.; Tan, C. T.; Chian, C. H.; Tan, C. H. Tetrahedron Lett. 2006, 47, 1007.[8] (a) Mori, A.; Abet, H.; Inoue, S. Appl. Organomet. Chem. 1995, 9, 189.(b) Paradowska, J.; Stodulski, M.; Mlynarski, J. Angew. Chem., Int. Ed. 2009, 48, 4288.[9] Urbanczyk-Lipkowska, B. Z.; KraJewski, J. W.; Gluzinski, P. Acta Crystallogr. 1982, B38, 971.[10] Yagi, Y.; Tanaka, I.; Yamane, T.; Ashida, T. J. Am. Chem. Soc. 1983, 105, 1242.[11] Martin, A. E.; Ford, T. M.; Bulkowski, J. E. J. Org. Chem. 1982, 47, 412.[12] Sheldrick, G. M. SHELXS-97, University of Göttingen, Göttingen, Germany, 1997. |