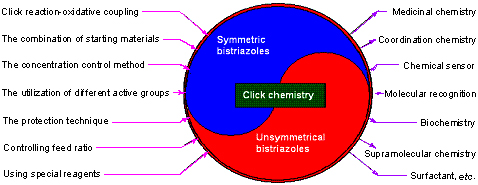
| [1] Meldal, M.; Tornoe, C. W. Chem. Rev. 2008, 108, 2952.[2] Bonger, K. M.; Hoogendoorn, S.; van Koppen, C. J.; Timmers, C. M.; van der Marel, G. A.; Overkleeft, H. S. ACS Med. Chem. Lett. 2011, 2, 85.[3] Chan, T. R.; Hilgraf, R.; Sharpless, K. B.; Fokin, V. V. Org. Lett. 2004, 6, 2853.[4] Wang, Q.; Chan, T. R.; Hilgraf, R.; Fokin, V. V.; Sharpless, K. B.; Finn, M. G. J. Am. Chem. Soc. 2003, 125, 3192.[5] Service, R. F. Science 2008, 320, 868.[6] Wu, P.; Feldman, A. K.; Nugent, A. K.; Hawker, C. J.; Scheel, A.; Voit, B.; Pyun, J.; Frechet, J. M. J.; Sharpless, K. B.; Fokin, V. V. Angew. Chem., Int. Ed. 2004, 43, 3928.[7] Iha, R. K.; Wooley, K. L.; Nystrom, A. M.; Burke, D. J.; Kade, M. J.; Hawker, C. J. Chem. Rev. 2009, 109, 5620.[8] Xiong, X.-Q.; Chen, H.-X. Chin. J. Org. Chem. 2013, 33, 1437 (in Chinese).(熊兴泉, 陈会新, 有机化学, 2013, 33, 1437.)[9] Sokolova, N. V.; Nenajdenko, V. G. RSC Adv. 2013, 3, 16212.[10] Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596.[11] Angell, Y.; Burgess, K. Angew. Chem., Int. Ed. 2007, 46, 3649.[12] Kwon, M.; Jang, Y.; Yoon, S.; Yang, D.; Jeon, H. B. Tetrahedron Lett. 2012, 53, 1606.[13] Gonzalez, J.; Perez, V. M.; Jimenez, D. O.; Lopez-Valdez G.; Corona, D.; Cuevas-Yanez, E. Tetrahedron Lett. 2011, 52, 3514.[14] Goyard, D.; Praly, J.-P.; Vidal, S. Carbohydr. Res. 2012, 362, 79.[15] Jur?ek, O.; Cametti, M.; Pontini, M.; Kolehmainena, E.; Rissanen, K. Org. Biomol. Chem. 2013, 11, 4585.[16] Lei, M.; Song, W.-Z.; Zhan, Z.-J.; Chen, X.-W.; Wang, Y.-G. Chin. J. Org. Chem. 2010, 30, 1854 (in Chinese).(雷鸣, 宋汪泽, 詹祖金, 陈小微, 王彦广, 有机化学, 2010, 30, 1854.)[17] Wang, Z.-X.; Zhao, Z.-G. J. Heterocycl. Chem. 2007, 44, 89.[18] Huang, Y.-E.; Gard, G.-L.; Shreeve, J.-M. Tetrahedron Lett. 2010, 51, 6951.[19] Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Chem. Rev. 2013, 113, 4905.[20] Oladeinde, O. A.; Hong, S. Y.; Holland, R. J.; Maciag, A. E.; Keefer, L. K.; Saavedra, J. E.; Nandurdikar, R. S. Org. Lett. 2010, 12, 4256.[21] Gaur, M.; Goel, M.; Sridhar, L.; Ashok, T. D. S.; Prabhakar, S.; Dureja, P.; Raghunathan, P.; Eswaran, S. V. Monatsh. Chem. 2012, 143, 283.[22] Huigens, R. W.; Rogers, S. A.; Steinhauer, A. T.; Melander, C. Org. Biomol. Chem. 2009, 7, 794.[23] Karuturi, R.; Al-Horani, R. A.; Mehta, S. C.; Gailani, D.; Desai, U. R. J. Med. Chem. 2013, 56, 2415.[24] Jurášeka, M.; D?ubákb, P.; Sedlákc, D.; Dvo?ákováa, H.; Hajdúchb, M.; Bart?někc, P.; Drašar, P. Steroids 2013, 78, 356.[25] Camp, C.; Dorbes, S.; Picard, C.; Benoist, E. Tetrahedron Lett. 2008, 49, 1979.[26] Hung, H.-C.; Cheng, C.-W.; Ho, I.-T.; Chung, W.-S. Tetrahedron Lett. 2009, 50, 302.[27] Hung, H.-C.; Cheng, C.-W.; Wang, Y.-Y.; Chen, Y.-J.; Chung, W.-S. Eur. J. Org. Chem. 2009, 6360.[28] Huang, H.-J.; Fang, H.-Y.; Chir, J.-L.; Wu, A.-T. Luminescence 2011, 26, 518.[29] Li, C.; Tang, J.; Xie, J. Tetrahedron 2009, 65, 7935.[30] Romero, T.; Orenes, R. A.; Tárraga, A.; Molina, P. Organo-metallics 2013, 32, 5740.[31] Wei, P.-F.; Yan, X.-Z.; Li, J.-Y.; Ma, Y.-J.; Yao, Y.; Huang, F.-H. Tetrahedron 2012, 68, 9179.[32] Ol'shevskaya, V. A.; Makarenkov, A. V.; Kononova, E. G.; Petrovskii, P. V.; Verbitskiy, E. V.; Rusinov, G. L.; Charushin, V. N.; Hey-Hawkins, E.; Kalinin, V. N. Polyhedron 2012, 42, 302.[33] Zhang, X. J.; Li, H. Y.; You, L. F.; Tang, Y.; Hsung, R. P. Adv. Synth. Catal. 2006, 348, 2437.[34] Sarmiento-Sánchez, J. I.; Ochoa-Teran, A.; Rivero, I. A. ARKIVOC 2011, 177.[35] Scott, S. O.; Gavey, E. L.; Lind, S. J.; Gordon, K. C.; Crowley, J. D. Dalton Trans. 2011, 40, 12117.[36] Guisado-Barrios, G.; Bouffard, J.; Donnadieu, B.; Bertrand, G. Organometallics 2011, 30, 6017.[37] Schweinfurth, D.; Büttner, N.; Hohloch, S.; Deibel, N.; Klein, J.; Sarkar, B. Organometallics 2013, 32, 5834.[38] Singh, N.; Metla, B. P. R.; Elias, A. J. J. Organomet. Chem. 2012, 717, 99.[39] Li, Y.-J.; Huffman, J. C.; Flood, A. H. Chem. Commun. 2007, 2692.[40] Fletcher, J. T.; Bumgarner, B. J.; Engels, N. D.; Skoglund, D. A. Organometallics 2008, 27, 5430.[41] Mulla, K.; Shaik, H.; Thompson, D. W.; Zhao, Y. M. Org. Lett. 2013, 15, 4532.[42] Li, C.; Henry, E.; Mani, N. K.; Tang, J.; Brochon, J. C.; Deprez, E.; Xie, J. Eur. J. Org. Chem. 2010, 2395.[43] Ruan, Y.-B.; Yu, Y.-H.; Li, C.; Bogliotti, N.; Tang, J.; Xie, J. Tetrahedron 2013, 69, 4603.[44] Brombosz, S. M.; Appleton, A. L.; Zappas, A. J.; Bunz, U. H. F. Chem. Commun. 2010, 46, 1419.[45] Shi, W.-J.; Liu, J.-Y.; Ng, D. K. P. Chem. Asian J. 2012, 7, 196.[46] Zhou, M.-Z.; Zhang, X.; Bai, M.-F.; Shen, D.-W.; Xu, B.-G.; Kao, J.; Ge, X.; Achilefu, X. RSC Adv. 2013, 3, 6756.[47] Wei, J.-J.; Jin, L.; Wan, K.; Zhou, C.-H. Bull. Korean Chem. Soc. 2011, 32, 229.[48] Lau, Y. H.; Rutledge, P. J.; Watkinson, M.; Todd, M. H. Chem. Soc. Rev. 2011, 40, 2848.[49] Hemamalinia, A.; Das, T. M. New J. Chem. 2013, 37, 2419.[50] Xu, H.-R.; Li, K.; Liu, Q.; Wu, T.-M.; Wang, M.-Q.; Hou, J.-T.; Huang, Z.; Xie, Y.-M.; Yu, X.-Q. Analyst 2013, 2329.[51] White, N. G.; Beer, P. D. Org. Biomol. Chem. 2013, 11, 1326.[52] Mohammed, A. I.; Abboud, Z. H.; Alghanimi, A. H. O. Tetrahedron Lett. 2012, 53, 5081.[53] Mekni, N.; Baklouti, A. J. Soc. Chim. Tunisie 2009, 11, 15.[54] Jervis, P. J.; Moulis, M.; Jukes, J. P.; Ghadbane, H.; Cox, L. R.; Cerundolo, V.; Besra, G. S. Carbohydr. Res. 2012, 356, 152.[55] Bedard, A.-C.; Collins, S. K. J. Am. Chem. Soc. 2011, 133, 19976.[56] Damodiran, M.; Muralidharan, D.; Perumal, P. T. Bioorg. Med. Chem. Lett. 2009, 19, 3611.[57] Lal, K.; Kumar, A.; Pavan, M. S.; Kaushik, C. P. Bioorg. Med. Chem. Lett. 2012, 22, 4353.[58] Singh, M. K.; Tilak, R.; Nath, G.; Awasthi, S. K.; Agarwal, A. Eur. J. Med. Chem. 2013, 63, 635.[59] Skarpos, H.; Osipov, S. N.; Vorob'eva, D. V.; Odinets, I. L.; Lork, E.; Roeschenthaler, G.-V. Org. Biomol. Chem. 2007, 5, 2361.[60] Pereira, G. R.; Santos, L. J.; Luduvico, I.; Alves, R. B.; de Freitas, R. P. Tetrahedron Lett. 2010, 51, 1022.[61] Rajesh, R.; Periyasami, G.; Raghunathan, R. Tetrahedron Lett. 2010, 51, 1896.[62] Megiatto, J. D.; Schuster, D. I. J. Am. Chem. Soc. 2008, 130, 12872.[63] Mames, I.; Wawrzyniak, U. E.; Wo?ny, M.; Bilewicz, R.; Korybut-Daszkiewicz, B. Dalton Trans. 2013, 42, 2382.[64] Mekni, N. H.; Baklouti, A. Heterocycles 2012, 85, 1727.[65] Yoshida, Y.; Takizawa, S.; Sasai, H. Tetrahedron Lett. 2011, 52, 6877.[66] Lin, H. N. Walsh, C. T. J. Am. Chem. Soc. 2004, 126, 13998.[67] Krim, J.; Taourirte, M.; Engels, J. W. Molecules 2012, 17, 179.[68] Huo, J.-P.; Lv, M.-X.; Wang, Z.-Y.; Li, Y.-Z. Chin. J. Chem. 2012, 30, 2411.[69] Mao, C.-X.; Wang, Z.-Y.; Tan, Y.-H.; Xue, F.-L. Chin. J. Org. Chem. 2011, 31, 1377 (in Chinese).(毛超旭, 汪朝阳, 谭越河, 薛福玲, 有机化学, 2011, 31, 1377.)[70] Mo, Y.-Q.; Wang, Z.-Y.; Mei, W.-J.; Fu, J.-H.; Tan, Y.-H.; Luo, S.-H. Monatsh. Chem. 2012, 143, 443.[71] Luo, S.-H.; Wang, Q.-F.; Wang, Z.-Y.; Peng P. Res. Chem. Intermed. 2013, 39, 2513.[72] Tan, Y.-H.; Li, J.-X.; Xue, F.-L.; Qi. J.; Wang, Z.-Y. Tetrahedron 2012, 68, 2827.[73] Huo, J. P.; Luo, J. C.; Wu, W.; Xiong, J. F.; Mo, G. Z.; Wang, Z. Y. Ind. Eng. Chem. Res. 2013, 52, 11850.[74] Kumar, K.; Sagar, S.; Esau, L.; Kaur, M.; Kumar, V. Eur. J. Med. Chem. 2012, 58, 153.[75] Kumar, K.; Carrere-Kremer, S.; Kremer, L.; Guerardel, Y.; Biot, C.; Kumar, V. Dalton Trans. 2013, 42, 1492.[76] Midya, G. C.; Paladhi, S.; Bhowmik, S.; Saha, S.; Dash, J. Org. Biomol. Chem. 2013, 11, 3057.[77] Elamari, H.; Jlalia, I.; Louet, C.; Herscovici, J.; Meganem, F.; Girard, C. Tetrahedron: Asymmetry 2010, 21, 1179.[78] Elamari, H.; Meganem, F.; Herscovici, J.; Girard, C. Tetrahedron Lett. 2011, 52, 658.[79] Elamari, H.; Slimi, R.; Chabot, G. G.; Quentin, L.; Scherman, D.; Girard, C. Eur. J. Med. Chem. 2013, 60, 360.[80] Isaacman, M. J.; Corigliano, E. M.; Theogarajan, L. S. Biomacro- molecules 2013, 14, 2996.[81] Zhou, Q. H.; Gui, J. H.; Pan, C.-M.; Albone, E.; Cheng, X.; Suh, E. M.; Grasso, L.; Ishihara, Y.; Baran, P. S. J. Am. Chem. Soc. 2013, 135, 12994.[82] Sapsford, K. E.; Algar, W. R.; Berti, L.; Gemmill, K. B.; Casey, B. J.; Oh, E.; Stewart, M. H.; Medintz, I. L. Chem. Rev. 2013, 113, 1904.[83] Beal, D. M.; Albrow, V. E.; Burslem, G.; Hitchen, L.; Fernandes, C.; Lapthorn, C.; Roberts, L. R.; Selby, M. D.; Jones, L. H. Org. Biomol. Chem. 2012, 10, 548.[84] Aucagne, V.; Leigh, D. A. Org. Lett. 2006, 8, 4505.[85] Fiandanese, V.; Bottalico, D.; Marchese, G.; Punzi, A.; Capuzzolo, F. Tetrahedron 2009, 65, 10573.[86] Aizpurua, J. M.; Azcune, I.; Fratila, R. M.; Balentova, E.; Sagartzazu-Aizpurua, M.; Miranda, J. I. Org. Lett. 2010, 12, 1584.[87] Doak, B. C.; Scanlon, M. J.; Simpson, J. S. Org. Lett. 2011, 13, 537.[88] Kamal, A.; Shankaraiah, N.; Reddy. C. R.; Prabhakar, S.; Markandeya, N.; Srivastava, H. K.; Sastry, G. N. Tetrahedron 2010, 66, 5498.[89] Kovalovs, A.; Novosjolova, I.; Bizdena, E.; Bizane, I.; Skardziute, L.; Kazlauskas, K.; Jursenas, S.; Turks, M. Tetrahedron Lett. 2013, 54, 850.[90] Potewar, T. M.; Petrova, K. T.; Barros, M. T. Carbohydr. Res. 2013, 79, 60.[91] Westermann, B.; Dörner, S.; Brauch, S.; Schaks, A.; Heinke, R.; Stark, S.; van Delft, F. L.; van Berkel, S. S. Carbohydr. Res. 2013, 371, 61.[92] Yuan, Z; Kuang, G.-C.; Clark, R. J.; Zhu, L. Org. Lett. 2012, 14, 2590.[93] Guiard, J.; Fiege, B.; Kitov, P. I.; Peters, T.; Bundle, D. R. Chem. Eur. J. 2011, 17, 7438.[94] Wang, Z.-Y.; Tan Y.-H.; Huo, J.-P.; Qi, J.; Li, D. In 12th International Symposium for Chinese Organic Chemists, Eds.: Zhai, H.-B.; Wang, W., Lanzhou University, Lanzhou, 2012, pp. 27.[95] Banday, A. H.; Arora, B. S.; Alam, M. S.; Kumar, H. M. S. Helv. Chim. Acta 2007, 90, 2368.[96] Arora, B. S.; Shafi, S.; Singh, S.; Ismail, T.; Kumar, H. M. S. Carbohydr. Res. 2008, 343, 139.[97] Yan, W.-M.; Wang, Q.-Y.; Chen, Y.-F.; Petersen, J. L.; Shi, X.-D. Org. Lett. 2010, 12, 3308. |