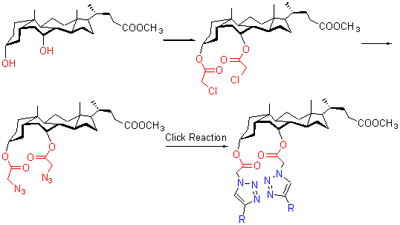
| [1] Clarkson. T. W. Am. J. Clin. Nutr. 1995, 61, 682.[2] Tchounwou, P. B.; Ayensu, W. K.; Ninashvili, N.; Sutton, D.; Environ. Toxicol. 2003, 18, 149.[3] An, L.; Cai, Y. H.; Yan, C. G. Chin. J. Appl. Chem. 2005, 22, 980 (in Chinese).(安琳, 蔡亚华, 颜朝国, 应用化学, 2005, 22, 980.)[4] Silva, A. P. de; Gunaratne, H. Q. N.; Gunnlaugsson, T.; Huxley, A. J. M.; McCoy, C. P.; Rademacher, J. T.; Rice, T. E. Chem. Rev. 1997, 97, 1515.[5] Kolb, H. C.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2001, 40, 2004.[6] Demko, Z. P.; Sharpless, K. B. Angew. Chem., Int. Ed., 2002, 41, 2110.[7] Lewis, W. G.; Green, L. G.; Grynszpan, F.; Radic, Z.; Carlier, P. R.; Taylor, P.; Finn, M. G.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 1053.[8] Schweinfurth, D.; Hardcastle, K. L.; Bunz, U. H. F. Chem. Commun. 2008, 1053.[9] Barreto, A. de F. S.; Vercillo, O. E.; Birkett, M. A.; Caulfield, J. C.; Wessjohann, L. A.; Andrade, C. K. Z. Org. Biomol. Chem. 2011, 9, 5024.[10] Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerova, M.; Praly, J.-P.; Imberty, A.; Vidal, S. Chem. Eur. J. 2011, 17, 2146.[11] Maeda, C.; Yamaguchi, S.; Ikeda, C.; Shinokubo, H.; Osaka, A. Org. Lett. 2008, 10, 549.[12] Wang, J.; He, X.; Gao, L.; Sheng, L.; Shi, X,; Li, J.; Chen, G. Chin. J. Chem. 2011, 29, 1227.[13] Fazio, M. A.; Lee, O. P.; Schuster, D. I. Org. Lett. 2008, 10, 4979.[14] Lowe, A. J.; Long, B. M.; Pfeffer, F. M. Chem. Commun. 2013, 49, 3376.[15] Oh, H.; Han, S. K.; Kim. B. H. Heteroat. Chem. 2012, 23, 187[16] Hu, J.; Lu, J. R.; Ju, Y. Chem. Asian J. 2011, 6, 2636.[17] Verzele, D.; Madder, A. Eur. J. Org. Chem. 2013, 673.[18] Jadhav, J. R.; Bae, C. H.; Kim. H. S. Tetrahedron Lett. 2011, 52, 1623.[19] Li, W. N.; Xu, Q.; Li, Y.; Zhu, W.; Cui, J. C.; Ju, Y.; Li, G. T. Tetrahedron Lett. 2013, 54, 3868.[20] Li, D. Z.; Yang, Y. X.; Yang, C.; Hu, B. W.; Huo, B. L.; Xue, L. W.; Wang, A. Z. Tetrahedron 2014, 70, 1223.[21] Hu, J.; Zhang, M.; Yu, L. B.; Ju. Y. Bioorg. Med. Chem. Lett. 2010, 20, 4342[22] Kumar, A.; Pandey, P. S. Tetrahedron Lett. 2009, 50, 5842.[23] Benesi, H. A.; Hildebrand, J. H. J. Am. Chem. Soc. 1949, 71, 2703. |