| [1] (a) Bruce, M. I. Chem. Rev. 1998, 98, 2797.
(b) Herndon, J. W. Coord. Chem. Rev. 2018, 356, 1 and references therein.
(c) Cadierno, V.; Gimeno, J. Chem. Rev. 2009, 109, 3512.
(d) Che, C. M.; Ho, C. M.; Huang, J. S. Coord. Chem. Rev. 2007, 251, 2145.
(e) Varela, J. A.; González-Rodríguez, C.; Saá, C. Top. Organ-omet. Chem. 2014, 48, 237.
(f) Lozano-Vila, A. M.; Monsaert, S.; Bajek, A.; Verpoort, F. Chem. Rev. 2010, 110, 4865.
(g) Nishibayashi, Y. Synthesis 2012, 44, 489.
(h) Rigaut, S.; Touchard, D.; Dixneuf, P. H.; Coord. Chem. Rev. 2004, 248, 1585.
(i) Bruneau, C.; Dixneuf, P. H. Angew. Chem., Int. Ed. 2006, 45, 2176.
[2] Selected examples:(a) Hyder, I.; Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2011, 30, 726.
(b) Smith, E. J.; Johnson, D. G.; Thatcher, R. J.; Whitwood, A. C.; Lynam, J. M. Organometallics 2013, 32, 7407.
(c) Bruce, M. I.; Burgun, A.; Fox, M. A.; Jevric, M.; Low, P. J.; Nicholson, B. K.; Parker, C. R.; Skelton, B. W.; White, A. H.; Zaitseva, N. N. Organometallics 2013, 32, 3286.
(d) Alós, J.; Bolaño, T.; Esteruelas, M. A.; Oliván, M.; Oñate, E.; Valencia, M. Inorg. Chem. 2014, 53, 1195.
(e) Garcia de la Arada, I.; Díez, J.; Gamasa, M. P.; Lastra, E. Organometallics 2015, 34, 1345.
(f) Schauer, P. A.; Skelton, B. W.; Koutsantonis, G. A. Organometallics 2015, 34, 4975.
(g) Spoerler, S.; Strinitz, F.; Rodehutskors, P.; Mueller, L.; Waterloo, A. R.; Duerr, M.; Huebner, E.; Ivanovic-Burmazovic, I.; Tykwinski, R. R.; Burzlaff, N. New J. Chem. 2016, 40, 2167.
(h) Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2016, 35, 388.
(i) Huang, J. B.; Zhou, X. X.; Zhao, Q. Y.; Li, S. H.; Xia, H. P. Chin. J. Chem. 2017, 35, 420.
[3] Coletti, C.; Marrone, A.; Re, N. Acc. Chem. Res. 2012, 45, 139.
[4] (a) Cadierno, V.; Gamasa, M. P.; Gimeno, J.; González-Cueva, M.; Lastra, E.; Borge, J.; García-Granda, S.; Pérez-Carreńo, E. Organometallics 1996, 15, 2137.
(b) Auger, N.; Touchard, D.; Rigaut, S.; Halet, J.-F.; Saillard, J.-Y. Organometallics 2003, 22, 1638.
(c) Wong, C.-Y.; Lai, L.-M.; Lam, C.-Y.; Zhu, N. Organometallics 2008, 27, 5806.
[5] Selected examples:(a) Pino-Chamorro, J. A.; Bustelo, E.; Puerta, M. C.; Valerga, P. Organometallics 2009, 28, 1546.
(b) Bustelo, E.; Jimenez-Tenorio, M.; Puerta, M. C.; Valerga, P. Organometallics 2007, 26, 4300.
(c) Rigaut, S.; Touchard, D.; Dixneuf, P. H. Organometallics 2003, 22, 3980.
(d) Bustelo, E.; Jiménez-Tenorio, M.; Mereiter, K.; Puerta, M. C.; Valerga, P. Organometallics 2002, 21, 1903.
[6] Selected examples:(a) Esteruelas, M. A.; Gómez, A. V.; López, A. M.; Oñate, E.; Ruiz, N. Organometallics 1999, 18, 1606.
(b) Bernad, D. J.; Esteruelas, M.; López, A. M.; Oliván, M.; Oñate, E.; Puerta, M. C.; Valerga, P. Organometallics 2000, 19, 4327.
(c) Kanao, K.; Tanabe, Y.; Miyake, Y.; Nishibayashi, Y. Organometallics 2010, 29, 2381.
(d) Queensen, M. J.; Rath, N. P.; Bauer, E. B. Organometallics 2014, 33, 5052.
(e) Strinitz, F.; Tucher, J.; Januszewski, J. A.; Waterloo, A. R.; Stegner, P.; Förtsch, S.; Hübner, E.; Tykwinski, R. R.; Burzlaff, N. Organometallics 2014, 33, 5129.
(f) Garcia de la Arada, I.; Díez, J.; Gamasa, M. P.; Lastra, E. J. Organomet. Chem. 2015, 797, 101.
[7] (a) Chen, K.-H.; Feng, Y. J.; Ma, H.-W.; Lin, Y.-C.; Liu, Y.-H.; Kuo, T.-S. Organometallics 2010, 29, 6829.
(b) Cadierno, V.; Conejero, S.; Gamasa, M. P.; Gimeno, J.; Falvello, L. R.; Llusar, R. M. Organometallics 2002, 21, 3716.
(c) Cadierno, V.; Gamasa, M. P.; Gimeno, J. Organometallics 1998, 17, 5216.
[8] (a) Serrano-Ruiz, M.; Lidrissi, C.; Mañas, S.; Peruzzini, M.; Romerosa, A. J. Organomet. Chem. 2014, 751, 654.
(b) Talavera, M.; Bolańo, S.; Bravo, J.; Castro, J.; García-Fontán, S.; Hermida-Ramón, J. M. Organometallics 2013, 32, 4402.
[9] (a) Venâncio, A. I. F. M.; Guedes da Silva, F. C.; Martins, L. M. D. R. S.; Fraústo da Silva, J. J. R.; Pombeiro, A. J. L. Organometallics 2005, 24, 4654.
(b) Cadierno, V.; Gamasa, M. P.; Gimeno, J.; López-González, M. C.; Borge, J.; Garciá-Granda, S. Organometallics 1997, 16, 4453.
[10] (a) Fischer, H.; Reindl, D.; Troll, C.; Leroux, F. J. Organomet. Chem. 1995, 490, 221.
(b) Esteruelas, M. A.; Gómez, A. V.; López, A. M.; Modriego, J.; Oñate, E. Organometallics 1998, 17, 5434.
(c) Bolańo, S.; Rodríguez-Rocha, M. M.; Bravo, J.; Castro, J.; Oñate, E.; Peruzzini, M. Organometallics 2009, 28, 6020.
(d) Jiménez-Tenorio, J.; Palacios, M. D.; Puerta, M. C.; Valerga, P. J. Organomet. Chem. 2004, 689, 2776.
(e) Peruzzini, M.; Barbaro, P.; Bertolasi, V.; Bianchini, C.; Mantovani, N.; Marvelli, L.; Rossi, R. Dalton Trans. 2003, 4121.
(f) Coletti, C.; Gonsalvi, L.; Guerriero, A.; Marvelli, L.; Peruzzini, M.; Reginato, G.; Re, N. Organometallics 2010, 29, 5982.
[11] Utegenov, K. I.; Krivykh, V. V. Glukhov, I. V. Petrovskii, P. V.; Ustynyuk, N. A. J. Organomet. Chem. 2011, 696, 3408.
[12] (a) Beletskaya, I. P.; Cheprakov, A. V. Organometallics 2012, 31, 7753.
(b) Hoover, J. M.; DiPasquale, A.; Mayer, J. M.; Michael, F. E. J. Am. Chem. Soc. 2010, 132, 5043.
(c) Schweizer, P. D.; Wadepohl, H.; Gade, L. H. Organometallics 2013, 32, 3697.
[13] (a) Barrett, A. G. M.; Carpenter, N. E.; Sabat, M. J. Organomet. Chem. 1988, 352, C8.
(b) Alt, H. G.; Engelhardt, H. E.; Steinlein, E.; Rogers, D. J. Organomet. Chem. 1987, 344, 321.
(c) Albertin, G.; Antoniutti, S.; Bortoluzzi, M.; Botter, A.; Castro, J. Dalton Trans. 2015, 44, 3439.
[14] Dabb, S. L.; Messerle, B. A.; Wagler, J. Organometallics 2008, 27, 4657.
[15] (a) Fukumoto, Y.; Dohi, T.; Masaoka, H.; Chatani, N.; Murai, S. Organometallics 2002, 21, 3845.
(b) Fukumoto, Y.; Tamura, Y.; Iyori, Y.; Chatani, N. J. Org. Chem. 2016, 81, 3161.
(c) Fukumoto, Y.; Ohmae, A.; Hirano, M.; Chatani, N. Asian J. Org. Chem. 2013, 2, 1036.
(d) Fukumoto, Y.; Asai, H.; Shimizu, M.; Chatani, N. J. Am. Chem. Soc. 2007, 129, 13792.
[16] Szesni, N.; Hohberger, C.; Mohamed, G. G.; Burzlaff, N.; Weibert, B.; Fischer, H. J. Organomet. Chem. 2006, 691, 5753.
[17] Cai, T.; Yang, Y.; Li, W.-W.; Qin, W.-B.; Wen, T.-B. Chem.-Eur. J. 2018, 24, 1606.
[18] Selegue, J. P. Organometallics 1982, 1, 217.
[19] (a) Aumann, R.; Jasper, B.; Fröhlich, R. Organometallics 1996, 14, 2447.
(b) Das, U. K.; Bhattacharjee, M. Chem.-Eur. J. 2012, 18, 5180.
(c) Sgro, M. J.; Stephan, D. W. Dalton Trans. 2013, 42, 10460.
[20] (a) Anil Kumar, P. G.; Pregosin, P. S.; Vallet, M.; Bernardinelli, G.; Jazzar, R. F.; Viton, F.; Kündig, E. P. Organometallics 2004, 23, 5410.
(b) Chiririwa, H.; Meijboom, R. Acta Crystallogr. 2011, E67, m1335.
[21] (a) Kopf, H.; Holzberger, B.; Pietraszuk, C.; Hübner, E.; Burzlaff, N. Organometallics 2008, 27, 5894.
(b) Jiménez-Tenorio, M.; Palacios, M. D.; Puerta, M. C.; Valerga, P. J. Organomet. Chem. 2004, 689, 2776.
(c) Bernad, D. J.; Esteruelas, M.; López, A. M.; Modrego, J.; Puerta, M. C.; Valerga, P. Organometallics 1999, 18, 4995.
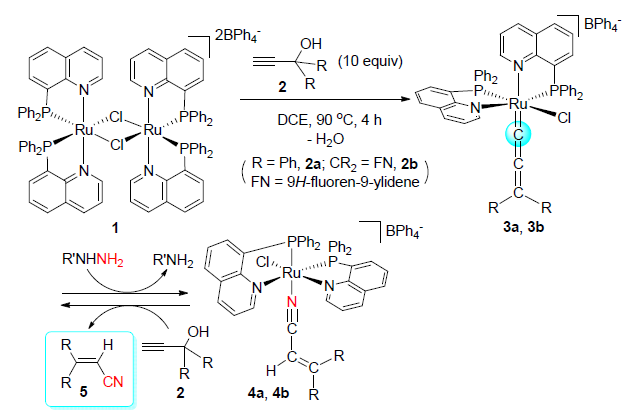
[22] In the catalytic reactions, benzophenone or 9H-fluoren-9-one was also isolated as the byproducts (ca. 25%). We initially envisioned that the H2O presented in the reaction solution, which released along with the Selegue's reaction during the formation of the allenylidene complex, might lead to the hydrolysis of the acrylonitrile product 5 to give the respective ketones. We have performed the control experiments by heating a solution of 3,3-diphenylacrylo-nitrile (5a) in DCE with purposely added water at even 110℃ for several hours with or without 2.5 mol% of complex 1. However, the hydrolysis issue is unlikely. As reflected by the TLC of the reaction solution, only trace amount of benzophenone can be detected. On the other hand, it has been reported that γ-substituted tert-propargyl alcohols have been involved in Sonogashira-type reactions as masked terminal alkynes via β-carbon elimination with liberation of ketone (see Ref.
[23] ). We tentatively envisioned that the ketone byproducts obtained in the catalytic reaction might come from β-carbon elimination of terminal tert-propargyl alcohols.
[23] (a) Nishimura, T.; Ariki, H.; Maeda, Y.; Uemura, S. Org. Lett. 2003, 5, 2997.
(b) Funayama, A.; Satoh, T.; Miura, M. J. Am. Chem. Soc. 2005, 127, 15354.
(c) Li, T.; Wang, Z.; Zhang, M. L.; Zhang H.-J.; Wen, T.-B. Chem. Commun. 2015, 51, 6777.
(d) Li, T.; Wang, Z.; Qin, W.-B.; Wen, T.-B. ChemCatChem 2016, 8, 2146.
[24] Hao, L.; Wu, F.; Ding, Z.-C.; Xu, S.-X.; Ma, Y.-L.; Chen, L.; Zhan, Z.-P. Chem.-Eur. J. 2012, 18, 6453.
[25] Chiarucci, M.; Mocci, R.; Syntrivanis, L. D.; Cera, G.; Mazzanti, A.; Bandini, M. Angew. Chem., Int. Ed. 2013, 52, 10850.
[26] Hu, M. Y.; Ni, C. F.; Li, L. C.; Han, Y. X.; Hu, J. B. J. Am. Chem. Soc. 2015, 137, 14496.
[27] Shipilovskikh, S. A.; Vaganov, V. Y.; Denisova, E. I.; Rubtsov, A. E.; Malkov, A. V. Org. Lett. 2018, 20, 728. |