有机化学 ›› 2023, Vol. 43 ›› Issue (7): 2528-2542.DOI: 10.6023/cjoc202210036 上一篇 下一篇
研究论文
朱玥a, 陈璐a, 赵静a, 孙庆荣a, 杨维清a, 付海燕b, 马梦林a,*( )
)
收稿日期:2022-10-28
修回日期:2022-12-02
发布日期:2023-02-14
通讯作者:
马梦林
基金资助:
Yue Zhua, Lu Chena, Jing Zhaoa, Qingrong Suna, Weiqing Yanga, Haiyan Fub, Menglin Maa( )
)
Received:2022-10-28
Revised:2022-12-02
Published:2023-02-14
Contact:
Menglin Ma
Supported by:文章分享
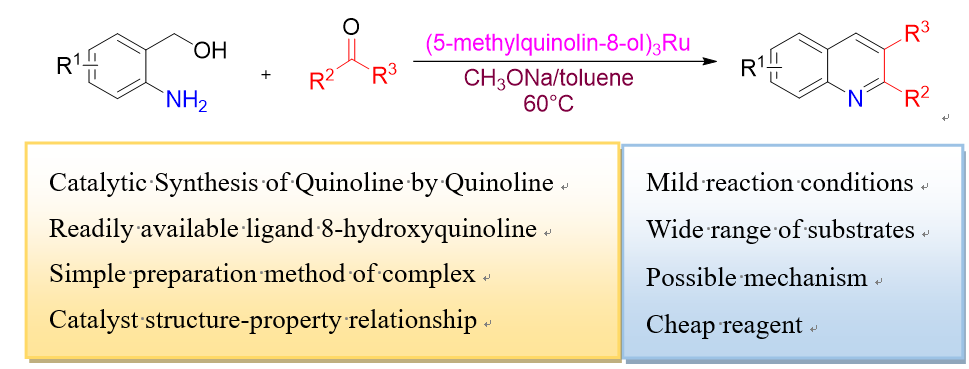
Friedländer喹啉合成法是以邻胺基芳基醛或酮与有α-亚甲基的酮环化制备喹啉的反应, 报道了一种喹啉钌络合物催化Friedländer法合成喹啉的方法. 首先, 以8-羟基喹啉钌络合物为催化剂, 对模板反应邻氨基苯甲醇和苯乙酮合成2-苯基喹啉进行了反应条件优化实验. 重点对比研究了8-羟基喹啉钌络合物配体上不同取代基对反应收率的影响, 其中5-甲基-8-羟基喹啉(1e)钌络合物催化邻氨基苯甲醇和苯乙酮合成2-苯基喹啉获得了73%的最高收率. 结合IR, UV以及密度泛函理论(DFT)计算讨论了配体结构与催化性能之间的关系. 提出了β-H消除形成醛过渡态, 交叉aldol反应再亚胺环化, 最后脱水生成目标产物的可行机理. 以(1e)3Ru为催化剂, 在优化的反应条件下进行了底物扩展研究, 以69%~94%的收率合成了32个不同取代的喹啉衍生物, 验证了方法的普适性.
朱玥, 陈璐, 赵静, 孙庆荣, 杨维清, 付海燕, 马梦林. 取代8-羟基喹啉钌络合物催化Friedländer反应合成喹啉衍生物[J]. 有机化学, 2023, 43(7): 2528-2542.
Yue Zhu, Lu Chen, Jing Zhao, Qingrong Sun, Weiqing Yang, Haiyan Fu, Menglin Ma. Synthesis of Quinoline Derivatives by Friedländer Reaction Catalyzed by Ruthenium Complexes of Substituted 8-Hydroxyquinoline[J]. Chinese Journal of Organic Chemistry, 2023, 43(7): 2528-2542.
| Entry | Cat. | Conversion rate/% | Selectivityb/% |
|---|---|---|---|
| 1 | — | 0 | 0c |
| 2 | (1a)3Al | 0 | 0c |
| 3 | (1a)2Cu | 12 | 37 |
| 4 | (1a)3Ir | 14 | 35 |
| 5 | (1a)2Pd | 16 | 35 |
| 6 | (1a)3Ru | 32 | 33 |
| Entry | Cat. | Conversion rate/% | Selectivityb/% |
|---|---|---|---|
| 1 | — | 0 | 0c |
| 2 | (1a)3Al | 0 | 0c |
| 3 | (1a)2Cu | 12 | 37 |
| 4 | (1a)3Ir | 14 | 35 |
| 5 | (1a)2Pd | 16 | 35 |
| 6 | (1a)3Ru | 32 | 33 |
| Entry | n(2a)∶n(3a) | Atmosphere | Base (equiv.) | Cat. (C/S) | Temp./℃ | Solvent | CR/% | Selectivitya/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1∶1.1 | Air | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 32 | 33 |
| 2 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 40 | 43 |
| 3 | 1∶1.1 | Ar | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 41 | 42 |
| 4 | 1∶1.1 | N2 | —b | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 0c | 0b |
| 5 | 1∶1.1 | N2 | NaOH (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 50 | 26 |
| 6 | 1∶1.1 | N2 | t-BuOK (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 40 | 33 |
| 7 | 1∶1.1 | N2 | KOH (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 47 | 29 |
| 8 | 1∶1.1 | N2 | K2CO3 (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 17 | 54 |
| 9 | 1∶1.1 | N2 | Et3N (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 2 | 1 |
| 10 | 1∶1.1 | N2 | Pyridine (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 2 | 1 |
| 11 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 82 | CH3CN | 29 | 44 |
| 12 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 67 | Tetrahydrofuran | 11 | 54 |
| 13 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 85 | tert-Butanol | 53 | 35 |
| 14 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 111 | Toluene | 57 | 51 |
| 15 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 102 | 1,4-Dioxane | 32 | 60 |
| 16 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 100 | DMSO | 22 | 24 |
| 17 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 100 | DMF | 1 | 2 |
| 18 | 1∶1.2 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 53 | 56 |
| 19 | 1∶1.3 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 58 | 56 |
| 20 | 1∶1.4 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 59 | 57 |
| 21 | 1∶1.5 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 61 | 57 |
| 22 | 1∶1.6 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 59 | 57 |
| 23 | 1∶2.0 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 50 | 57 |
| 24 | 1∶1.2 | N2 | CH3ONa (1.1) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 53 | 55 |
| 25 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 68 | 62 |
| 26 | 1∶1.2 | N2 | CH3ONa (1.4) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 67 | 61 |
| 27 | 1∶1.2 | N2 | CH3ONa (1.5) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 51 | 59 |
| 28 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.2 mol%) | 110 | Toluene | 67 | 63 |
| 29 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.3 mol%) | 110 | Toluene | 69 | 63 |
| 30 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.4 mol%) | 110 | Toluene | 70 | 63 |
| 31 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 110 | Toluene | 73 | 64 |
| 32 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (1.0 mol%) | 110 | Toluene | 75 | 65 |
| 33 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (2.0 mol%) | 110 | Toluene | 74 | 65 |
| 34 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 20 | Toluene | 0b | 0b |
| 35 | 1∶1.52 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 40 | Toluene | 64 | 63 |
| 36 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 60 | Toluene | 74 | 70 |
| 37 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 90 | Toluene | 77 | 64 |
| Entry | n(2a)∶n(3a) | Atmosphere | Base (equiv.) | Cat. (C/S) | Temp./℃ | Solvent | CR/% | Selectivitya/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 1∶1.1 | Air | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 32 | 33 |
| 2 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 40 | 43 |
| 3 | 1∶1.1 | Ar | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 41 | 42 |
| 4 | 1∶1.1 | N2 | —b | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 0c | 0b |
| 5 | 1∶1.1 | N2 | NaOH (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 50 | 26 |
| 6 | 1∶1.1 | N2 | t-BuOK (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 40 | 33 |
| 7 | 1∶1.1 | N2 | KOH (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 47 | 29 |
| 8 | 1∶1.1 | N2 | K2CO3 (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 17 | 54 |
| 9 | 1∶1.1 | N2 | Et3N (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 2 | 1 |
| 10 | 1∶1.1 | N2 | Pyridine (1.2) | (1a)3Ru (0.1 mol%) | 70 | CH3OH | 2 | 1 |
| 11 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 82 | CH3CN | 29 | 44 |
| 12 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 67 | Tetrahydrofuran | 11 | 54 |
| 13 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 85 | tert-Butanol | 53 | 35 |
| 14 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 111 | Toluene | 57 | 51 |
| 15 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 102 | 1,4-Dioxane | 32 | 60 |
| 16 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 100 | DMSO | 22 | 24 |
| 17 | 1∶1.1 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 100 | DMF | 1 | 2 |
| 18 | 1∶1.2 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 53 | 56 |
| 19 | 1∶1.3 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 58 | 56 |
| 20 | 1∶1.4 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 59 | 57 |
| 21 | 1∶1.5 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 61 | 57 |
| 22 | 1∶1.6 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 59 | 57 |
| 23 | 1∶2.0 | N2 | CH3ONa (1.2) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 50 | 57 |
| 24 | 1∶1.2 | N2 | CH3ONa (1.1) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 53 | 55 |
| 25 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 68 | 62 |
| 26 | 1∶1.2 | N2 | CH3ONa (1.4) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 67 | 61 |
| 27 | 1∶1.2 | N2 | CH3ONa (1.5) | (1a)3Ru (0.1 mol%) | 110 | Toluene | 51 | 59 |
| 28 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.2 mol%) | 110 | Toluene | 67 | 63 |
| 29 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.3 mol%) | 110 | Toluene | 69 | 63 |
| 30 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.4 mol%) | 110 | Toluene | 70 | 63 |
| 31 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 110 | Toluene | 73 | 64 |
| 32 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (1.0 mol%) | 110 | Toluene | 75 | 65 |
| 33 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (2.0 mol%) | 110 | Toluene | 74 | 65 |
| 34 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 20 | Toluene | 0b | 0b |
| 35 | 1∶1.52 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 40 | Toluene | 64 | 63 |
| 36 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 60 | Toluene | 74 | 70 |
| 37 | 1∶1.2 | N2 | CH3ONa (1.3) | (1a)3Ru (0.5 mol%) | 90 | Toluene | 77 | 64 |
| Entry | Cat. | Positions of substituted | CR/% | Selectivityb/% |
|---|---|---|---|---|
| 1 | (1a)3Ru | —c | 74 | 70 |
| 2 | (1c)3Ru | 2-Substituted quinoline | 17 | 18 |
| 3 | (1d)3Ru | 2-Substituted quinoline | 45 | 20 |
| 4 | (1g)3Ru | 2-Substituted quinoline | 64 | 13 |
| 5 | (1h)3Ru | 2-Substituted quinoline | 52 | 16 |
| 6 | (1r)3Ru | 2-Substituted quinoline | 63 | 11 |
| 7 | (1s)3Ru | 2-Substituted quinoline | 54 | 20 |
| 8 | (1b)3Ru | 7-Substituted quinoline | 35 | 33 |
| 9 | (1l)3Ru | 7-Substituted quinoline | 44 | 25 |
| 10 | (1m)3Ru | 7-Substituted quinoline | 52 | 38 |
| 11 | (1i)3Ru | 3- or 4-Substituted quinoline | 69 | 65 |
| 12 | (1j)3Ru | 3- or 4-Substituted quinoline | 75 | 72 |
| 13 | (1p)3Ru | 3- or 4-Substituted quinoline | 75 | 67 |
| 14 | (1q)3Ru | 3- or 4-Substituted quinoline | 68 | 73 |
| 15 | (1e)3Ru | 5-Substituted quinoline | 81 | 96 |
| 16 | (1k)3Ru | 5-Substituted quinoline | 78 | 92 |
| 17 | (1n)3Ru | 5-Substituted quinoline | 58 | 85 |
| 18 | (1o)3Ru | 5-Substituted quinoline | 52 | 90 |
| 19 | (1f)3Ru | 5-Substituted quinoline | 94 | 26 |
| Entry | Cat. | Positions of substituted | CR/% | Selectivityb/% |
|---|---|---|---|---|
| 1 | (1a)3Ru | —c | 74 | 70 |
| 2 | (1c)3Ru | 2-Substituted quinoline | 17 | 18 |
| 3 | (1d)3Ru | 2-Substituted quinoline | 45 | 20 |
| 4 | (1g)3Ru | 2-Substituted quinoline | 64 | 13 |
| 5 | (1h)3Ru | 2-Substituted quinoline | 52 | 16 |
| 6 | (1r)3Ru | 2-Substituted quinoline | 63 | 11 |
| 7 | (1s)3Ru | 2-Substituted quinoline | 54 | 20 |
| 8 | (1b)3Ru | 7-Substituted quinoline | 35 | 33 |
| 9 | (1l)3Ru | 7-Substituted quinoline | 44 | 25 |
| 10 | (1m)3Ru | 7-Substituted quinoline | 52 | 38 |
| 11 | (1i)3Ru | 3- or 4-Substituted quinoline | 69 | 65 |
| 12 | (1j)3Ru | 3- or 4-Substituted quinoline | 75 | 72 |
| 13 | (1p)3Ru | 3- or 4-Substituted quinoline | 75 | 67 |
| 14 | (1q)3Ru | 3- or 4-Substituted quinoline | 68 | 73 |
| 15 | (1e)3Ru | 5-Substituted quinoline | 81 | 96 |
| 16 | (1k)3Ru | 5-Substituted quinoline | 78 | 92 |
| 17 | (1n)3Ru | 5-Substituted quinoline | 58 | 85 |
| 18 | (1o)3Ru | 5-Substituted quinoline | 52 | 90 |
| 19 | (1f)3Ru | 5-Substituted quinoline | 94 | 26 |
| Entry | Compd. | IR/cm-1 | UV/nm | Catalytic performance | ||
|---|---|---|---|---|---|---|
| Ru—O | C—O | CR/% | Selectivity/% | |||
| 1 | (1a)3Ru | 3372 | 527 | 431 | 74 | 70 |
| 2 | (1b)3Ru | 3438 | 538 | 429 | 35 | 33 |
| 3 | (1c)3Ru | 3286 | 540 | 428 | 17 | 18 |
| 4 | (1e)3Ru | 3439 | 542 | 444 | 81 | 96 |
| 5 | (1k)3Ru | 3444 | 548 | 443 | 78 | 92 |
| Entry | Compd. | IR/cm-1 | UV/nm | Catalytic performance | ||
|---|---|---|---|---|---|---|
| Ru—O | C—O | CR/% | Selectivity/% | |||
| 1 | (1a)3Ru | 3372 | 527 | 431 | 74 | 70 |
| 2 | (1b)3Ru | 3438 | 538 | 429 | 35 | 33 |
| 3 | (1c)3Ru | 3286 | 540 | 428 | 17 | 18 |
| 4 | (1e)3Ru | 3439 | 542 | 444 | 81 | 96 |
| 5 | (1k)3Ru | 3444 | 548 | 443 | 78 | 92 |
| Entry | Compd. | Bond length/nm | θ/(°) | HOMO/eV | LOMO/eV | HOMO(α)-LOMO(α) gap/eV | Catalytic performance | ||
|---|---|---|---|---|---|---|---|---|---|
| Ru—O | Ru—N | CR/% | Selectivity/% | ||||||
| 1 | (1a)3Ru | 0.203 | 0.210 | 80.4 | -6.30467 | -0.67078 | 5.63389 | 74 | 70 |
| 2 | (1b)3Ru | 0.204 | 0.210 | 81.9 | -6.13045 | -0.66572 | 5.46473 | 35 | 33 |
| 3 | (1c)3Ru | 0.200 | 0.215 | 81.4 | -6.12713 | -0.66283 | 5.46430 | 17 | 18 |
| 4 | (1e)3Ru | 0.200 | 0.210 | 80.4 | -6.33766 | -0.63527 | 5.70239 | 81 | 96 |
| 5 | (1k)3Ru | 0.200 | 0.210 | 80.3 | -6.32899 | -0.62135 | 5.70764 | 78 | 92 |
| Entry | Compd. | Bond length/nm | θ/(°) | HOMO/eV | LOMO/eV | HOMO(α)-LOMO(α) gap/eV | Catalytic performance | ||
|---|---|---|---|---|---|---|---|---|---|
| Ru—O | Ru—N | CR/% | Selectivity/% | ||||||
| 1 | (1a)3Ru | 0.203 | 0.210 | 80.4 | -6.30467 | -0.67078 | 5.63389 | 74 | 70 |
| 2 | (1b)3Ru | 0.204 | 0.210 | 81.9 | -6.13045 | -0.66572 | 5.46473 | 35 | 33 |
| 3 | (1c)3Ru | 0.200 | 0.215 | 81.4 | -6.12713 | -0.66283 | 5.46430 | 17 | 18 |
| 4 | (1e)3Ru | 0.200 | 0.210 | 80.4 | -6.33766 | -0.63527 | 5.70239 | 81 | 96 |
| 5 | (1k)3Ru | 0.200 | 0.210 | 80.3 | -6.32899 | -0.62135 | 5.70764 | 78 | 92 |
| [1] |
Zhu, Y.; Cai, C. RSC Adv. 2014, 4, 52911.
doi: 10.1039/C4RA07858F |
| [2] |
Pothikumar, R.; Bhat, V. T.; Namitharan, K. Chem. Commun. 2020, 56, 13607.
doi: 10.1039/D0CC05912A |
| [3] |
Akbari, J.; Heydari, A.; Kalhor, H. R.; Kohan, S. A. Cheminform 2010, 41, 137.
|
| [4] |
Das, S.; Sinha, S.; Samanta, D.a; Mondal, R.; Chakraborty, G.; Brandao, P.; Paul, N. D. J. Org. Chem. 2019, 84, 10160.
doi: 10.1021/acs.joc.9b01343 |
| [5] |
Mondal, R.; Chakraborty, G.; Guin, A. K.; Pal, S.; Paul, N. D. Tetrahedron 2021, 100, 132479.
doi: 10.1016/j.tet.2021.132479 |
| [6] |
(a) Genc, S.; Arslan, B.; Gulcemal, S.; Gunnaz, S.; Cetinkaya, B.; Gulcemal, D. J. Org. Chem. 2019, 84, 6286.
doi: 10.1021/acs.joc.9b00632 |
|
(b) Wang, R.; Fan, H.; Zhao, W.; Li, F. Org. Lett. 2016, 18, 3558.
doi: 10.1021/acs.orglett.6b01518 |
|
| [7] |
Das, S.; Maiti, D.; Sarkar, D. S. J. Org. Chem. 2018, 83, 2309.
doi: 10.1021/acs.joc.7b03198 |
| [8] |
Zhang, G.; Wu, J.; Zeng, H.; Zhang, S.; Yin, Z.; Zheng, S. Org. Lett. 2017, 19, 1080.
doi: 10.1021/acs.orglett.7b00106 |
| [9] |
Cho, C. S.; Seok, H. J.; Shim, S. C. J. Heterocycl. Chem. 2005, 42, 1219.
doi: 10.1002/jhet.v42:6 |
| [10] |
Mahajan, A.; Arya, A.; Chundawat, T. S. Synth. Commun. 2019, 49, 1926.
doi: 10.1080/00397911.2019.1610776 |
| [11] |
(a) Mierde, H. V.; Voort, P. V. D.; Vos, D. D.; Verpoort, F. Eur. J. Org. Chem. 2008, 1625.
|
|
(b) Mierde, H. V.; Ldoux, N.; Allaert, B.; Voort, P. V. D.; Drozdzak, R.; Vos, D. D.; Verpoort, F. New J. Chem. 2007, 31, 1572.
doi: 10.1039/b707292a |
|
| [12] |
Yun, X. J.; Zhu, J. W.; Yan, J. Deng, W.; Yao, Z. J. Inorg. Chem. 2020, 59, 7841.
doi: 10.1021/acs.inorgchem.0c00955 |
| [13] |
Huo, S.; Kong, S.; Zeng, G.; Feng, Q.; Hao, Z.; Han, Z.; Lin, J.; Lu, G. L. J. Mol. Catal. A: Chem. 2021, 514, 111773.
|
| [14] |
Verma, A.; Hazra, S.; Dolui, P.; Elias, A. J. J. Org. Chem. 2021, 10, 626.
|
| [15] |
(a) Zhang, Y.; Cheng, H.; Sun, Q.; Chen, H.; Yang, W.; Ma, M. J. Chem. Res. 2021, 45, 623.
doi: 10.1177/1747519820973601 |
|
(b) He, J.; Zhou, T.; Cao, Y.; Zhang, Y.; Yang, W.; Ma, M. J. Fluoresc. 2018, 28, 1121.
doi: 10.1007/s10895-018-2275-7 |
|
|
(c) Alam, M. N.; Moni, M. A.; Yu, J. Q; Beale, P.; Turner, P.; Proschogo, N.; Rahman, M. A.; Hossain, M. P.; Huq, P. Int. J. Mol. Sci. 2021, 22, 8471.
doi: 10.3390/ijms22168471 |
|
| [16] |
Rodman, G. S.; Nagle, J. K. Inorg. Chim. Acta 1985, 105, 205.
doi: 10.1016/S0020-1693(00)85231-7 |
| [17] |
(a) Vander, M. H.; Voot, P. V. D.; Vos, D. D.; Verpoort, F. Eur. J. Org. Chem. 2008, 1625.
|
|
(b) Subramanian, M.; Sundar, S.; Rengan, R. Appl. Organomet. Chem. 2018, 32, e4582.
|
|
| [18] |
Ghosh, T. N.; Lasker, S. L.; Banerjee, S. J. Indian Chem. Soc. 1944, 21, 354.
|
| [19] |
Bronson, R. T.; Montalti, M.; Prodi, L.; Zaccheroni, N.; Lamb, R. D.; Dalley, N. K.; Izatt, R. M.; Bradshaw, J. S.; Savage, P. B. Tetrahedron 2004, 60, 11139.
doi: 10.1016/j.tet.2004.08.062 |
| [20] |
Khusnutdinov, R. I.; Bayguzina, A. R.; Aminov, R. I. Russ. J Gen. Chem. 2016, 86, 1613.
doi: 10.1134/S1070363216070136 |
| [21] |
Thinnes, C. C.; Tumber, A.; Yapp, C.; Scozzafava, G.; Yeh, T.; Chan, M. C.; Tran, T. A.; Hsu, K.; Tarhonskaya, H.; Walport, L. J.; Wilkins, S. E.; Martinez, E. D.; Muller, S.; Pugh, C. W.; Ratcliffe, P. J.; Brennan, P. E.; Kawamura, A.; Schofield, C. J. Chem. Commun. 2015, 51, 15458.
doi: 10.1039/C5CC06095H |
| [22] |
Phillips, J. P.; Elbinger, R. L.; Merritt, L. L. J. Am. Chem. Soc. 1949, 71, 3986.
doi: 10.1021/ja01180a031 |
| [23] |
Warner, V. D.; Sane, J. N.; Mirth, D. B.; Turesky, S. S.; Soloway, B. J. Med. Chem. 1976, 19, 167.
pmid: 812992 |
| [24] |
Mirkovic, B.; Renko, M.; Turk, S.; Sosic, I.; Jevnikar, Z.; Obermajer, N.; Turk, D.; Gobec, S.; Kos, J. ChemMedChem 2011, 6, 1351.
doi: 10.1002/cmdc.v6.8 |
| [25] |
Lauer, W. M.; Arnold, R. T.; Tiffany, B.; Tinker, J. J. Am. Chem. Soc. 1946, 68, 1268.
doi: 10.1021/ja01211a040 |
| [26] |
Parua, S.; Sikari, R.; Sinha, S.; Das, S.; Chakraborty, G.; Paul, N. D. Org. Biomol. Chem. 2018, 16, 274.
doi: 10.1039/C7OB02670F |
| [27] |
Xi, L; Zhang, R.; Zhang, L.; Chen, S.; Yu, X. Org. Biomol. Chem. 2015, 13, 3924.
doi: 10.1039/C5OB00075K |
| [28] |
Xu, T.; Shao, Y.; Dai, L.; Yu, S.; Cheng, T.; Chen, J. J. Org. Chem. 2019, 84, 13604.
doi: 10.1021/acs.joc.9b01875 |
| [29] |
Saxena, J. P.; Stafford, W. H.; Stafford, W. L. J. Chem. Soc. 1959, 1579.
|
| [30] |
Qu, F; He, P.; Hu, R.; Cheng, X.; Wang, S.; Wu, J. Synth. Commun. 2015, 45, 2802.
doi: 10.1080/00397911.2015.1105982 |
| [31] |
Xu, J.; Sun, J.; Zhao, J.; Huang, B.; Li, X.; Sun, Y. RSC Adv. 2017, 7, 36242.
doi: 10.1039/C7RA06425J |
| [1] | 李洋, 董亚楠, 李跃辉. 经由N-硼基酰胺中间体的酰胺高效转化合成腈类化合物[J]. 有机化学, 2024, 44(2): 638-643. |
| [2] | 李思达, 崔鑫, 舒兴中, 吴立朋. 钛催化的烯烃制备1,1-二硼化合物[J]. 有机化学, 2024, 44(2): 631-637. |
| [3] | 邹发凯, 王能中, 姚辉, 王慧, 刘明国, 黄年玉. 1β-/3R-芳基硫代糖的区域与立体选择性合成[J]. 有机化学, 2024, 44(2): 593-604. |
| [4] | 刘继宇, 李圣玉, 陈款, 朱茵, 张元. 三苯胺功能化有序介孔聚合物作为无金属光催化剂用于二硫化物合成[J]. 有机化学, 2024, 44(2): 605-612. |
| [5] | 杨爽, 房新强. 氮杂环卡宾催化实现的动力学拆分近期研究进展[J]. 有机化学, 2024, 44(2): 448-480. |
| [6] | 李路瑶, 贺忠文, 张振国, 贾振华, 罗德平. 三芳基碳正离子在有机合成中的应用[J]. 有机化学, 2024, 44(2): 421-437. |
| [7] | 陈宛婷, 钟雄威, 邢佳乐, 吴昌书, 高杨. C—N轴手性化合物的不对称催化合成研究进展[J]. 有机化学, 2024, 44(2): 349-377. |
| [8] | 黄净, 杨毅华, 张占辉, 刘守信. 酰胺键的绿色高效构建方法与技术进展[J]. 有机化学, 2024, 44(2): 409-420. |
| [9] | 梅青刚, 李清寒. 可见光促进C(3)(杂)芳硫基吲哚化合物的合成研究进展[J]. 有机化学, 2024, 44(2): 398-408. |
| [10] | 朱彦硕, 王红言, 舒朋华, 张克娜, 王琪琳. 烷氧自由基引发1,5-氢原子转移实现C(sp3)—H键官能团化的研究进展[J]. 有机化学, 2024, 44(1): 1-17. |
| [11] | 赵茜帆, 陈永正, 张世明. 碳基非金属催化剂在有机合成领域的应用及机理研究[J]. 有机化学, 2024, 44(1): 137-147. |
| [12] | 赵红琼, 于淼, 宋冬雪, 贾琦, 刘颖杰, 季宇彬, 许颖. 羧酸脱羧羟基化反应研究进展[J]. 有机化学, 2024, 44(1): 70-84. |
| [13] | 金玉坤, 任保轶, 梁福顺. 可见光介导的三氟甲基的选择性C-F键断裂及其在偕二氟类化合物合成中的应用[J]. 有机化学, 2024, 44(1): 85-110. |
| [14] | 姜权彬. 经由氮杂邻联烯醌中间体合成轴手性化合物的研究进展[J]. 有机化学, 2024, 44(1): 159-172. |
| [15] | 文思, 丁宇浩, 田青于, 葛进, 程国林. 铑(III)催化苯甲亚胺酸乙酯和CF3-亚胺氧锍叶立德C—H 活化/环化反应合成CF3-1H-苯并[de][1,8]萘吡啶[J]. 有机化学, 2024, 44(1): 291-300. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||