Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (6): 1811-1819.DOI: 10.6023/cjoc202112041 Previous Articles Next Articles
ARTICLES
马丽文, 魏晓叶, 赵紫琳, 赵昂, 邓祥文, 霍丙南, 马刚, 张春芳*( )
)
收稿日期:2021-12-30
修回日期:2022-01-25
发布日期:2022-02-17
通讯作者:
张春芳
基金资助:
Liwen Ma, Xiaoye Wei, Zilin Zhao, Ang Zhao, Xiangwen Deng, Bingnan Huo, Gang Ma, Chunfang Zhang( )
)
Received:2021-12-30
Revised:2022-01-25
Published:2022-02-17
Contact:
Chunfang Zhang
Supported by:Share
Liwen Ma, Xiaoye Wei, Zilin Zhao, Ang Zhao, Xiangwen Deng, Bingnan Huo, Gang Ma, Chunfang Zhang. Theoretical Study on the Catalytic Mechanism of Copper with Various Valence for the Terminal Alkyne Coupling Reaction[J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1811-1819.
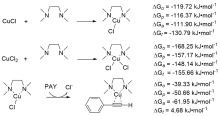
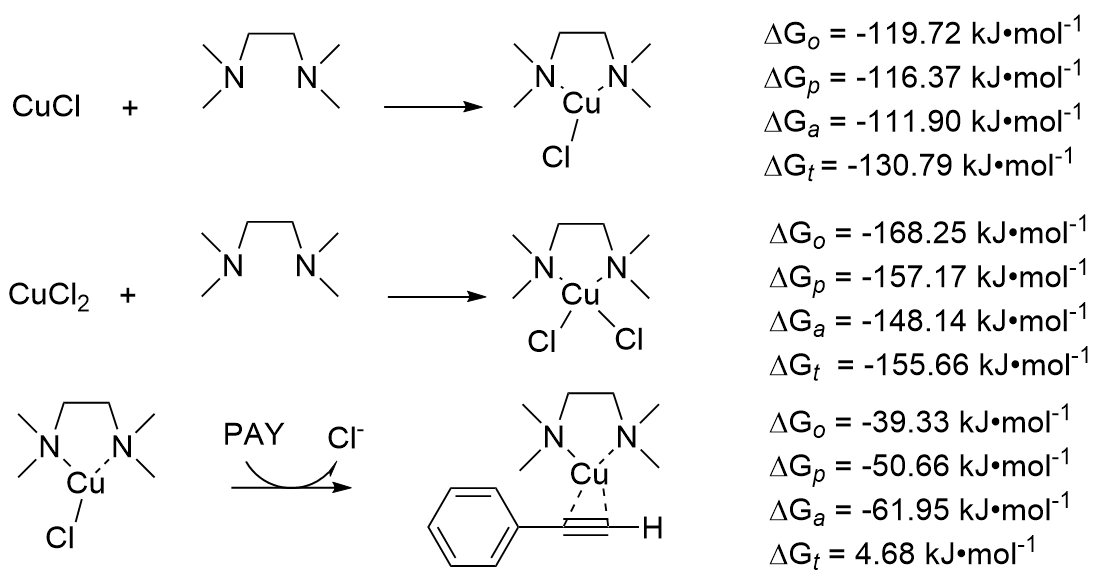
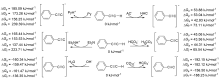
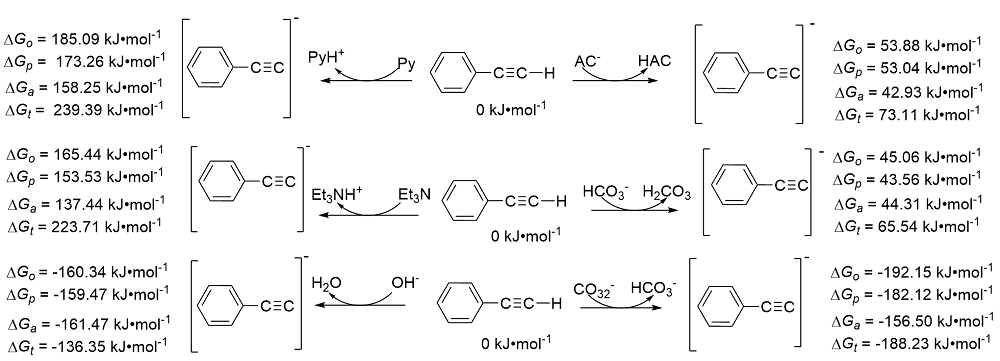

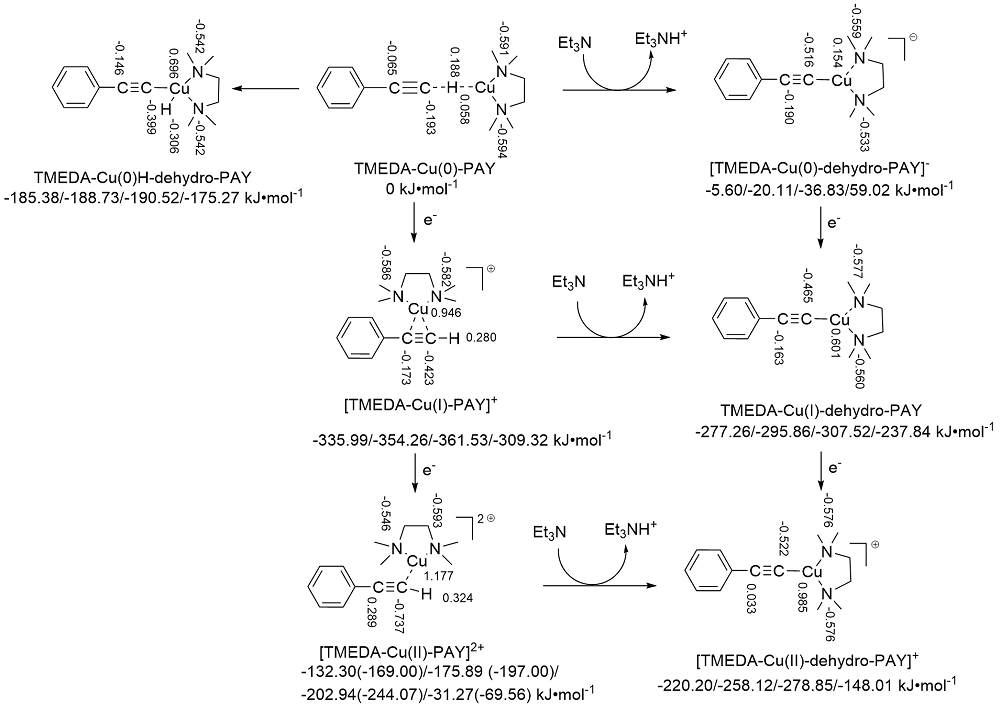
| Species | ERED/V | |||
|---|---|---|---|---|
| ODCB | Py | AC | CHCl3 | |
| [TMEDA-Cu(I)-PAY]+ | 0.095 | 0.092 | 0.089 | 0.11 |
| TMEDA-Cu(I)-dehydro-PAY | –0.028 | –0.023 | –0.018 | –0.048 |
| [TMEDA-Cu(II)-PAY]2+ | 0.90 | 0.90 | 0.89 | 0.94 |
| [TMEDA-Cu(II)-dehydro-PAY]+ | 0.53 | 0.53 | 0.53 | 0.55 |
| Species | ERED/V | |||
|---|---|---|---|---|
| ODCB | Py | AC | CHCl3 | |
| [TMEDA-Cu(I)-PAY]+ | 0.095 | 0.092 | 0.089 | 0.11 |
| TMEDA-Cu(I)-dehydro-PAY | –0.028 | –0.023 | –0.018 | –0.048 |
| [TMEDA-Cu(II)-PAY]2+ | 0.90 | 0.90 | 0.89 | 0.94 |
| [TMEDA-Cu(II)-dehydro-PAY]+ | 0.53 | 0.53 | 0.53 | 0.55 |
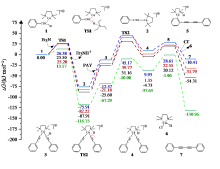

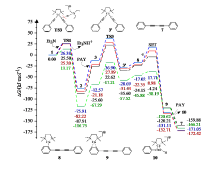
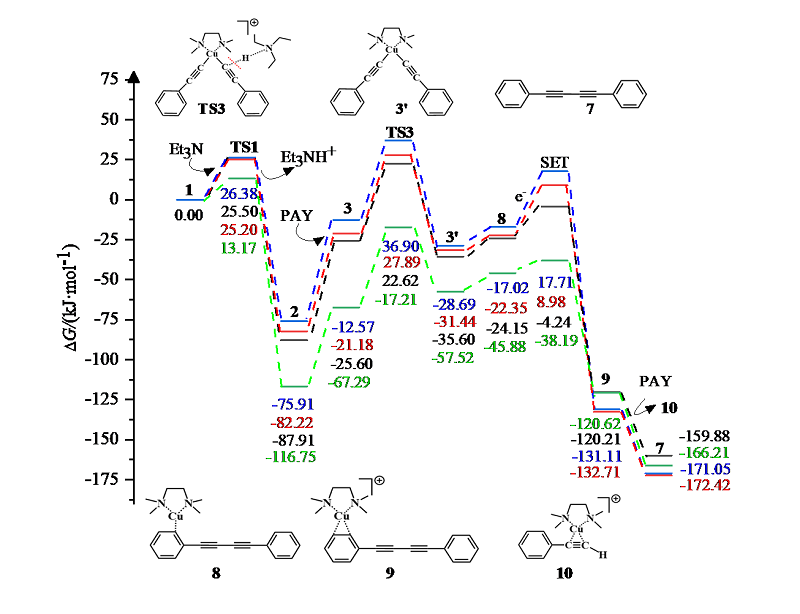
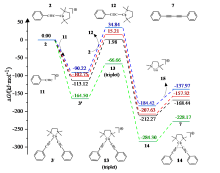

| [1] |
Roh, S. W.; Choi, K.; Lee, C. Chem. Rev. 2019, 119, 4293.
doi: 10.1021/acs.chemrev.8b00568 |
| [2] |
Li, Y. J.; Xu, L.; Liu, H. B.; Li, Y. L. Chem. Soc. Rev. 2014, 43, 2572.
doi: 10.1039/c3cs60388a |
| [3] |
He, J. J.; Wang, N.; Cui, Z. L.; Du, H. P.; Fu, L.; Huang, C. S.; Yang, Z.; Shen, X. Y.; Yi, Y. P.; Tu, .Z. Y.; Li, Y. L. Nat. Commun. 2017, 8, 1172.
doi: 10.1038/s41467-017-01202-2 |
| [4] |
Yu, J. M.; Liu, L.; Xu, B. M.; Liu, Z. Y; Shan, S. X. Acta Chim. Sinica 1996, 54, 922. (in Chinese)
|
|
( 余俊梅, 刘林, 徐保明, 刘智勇, 单书香, 化学学报, 1996, 54, 922.)
|
|
| [5] |
Ma, N.; Zeng, X. H. Chin. J. Org. Chem. 2018, 38, 1556. (in Chinese)
doi: 10.6023/cjoc201712038 |
|
( 马楠, 曾祥华, 有机化学, 2018, 38, 1556.)
doi: 10.6023/cjoc201712038 |
|
| [6] |
Zhang, C. X.; Li, N. N.; Li, X.; Chang, H. H.; Liu, Q.; Wei, W. L. Chin. J. Org. Chem. 2014, 34, 81. (in Chinese)
doi: 10.6023/cjoc201307024 |
|
( 张聪霞, 李娜娜, 李兴, 常宏宏, 刘强, 魏文珑, 有机化学, 2014, 34, 81.)
doi: 10.6023/cjoc201307024 |
|
| [7] |
Sun, S. J.; Bao, Y. S.; Zhaori, G. T. J. Mol. Catal. 2013, 27, 585. (in Chinese)
|
|
( 孙淑君, 包永胜, 照日格图, 分子催化, 2013, 27, 585.)
|
|
| [8] |
Zhang, M.; Wang, L. L.; Li, L. C.; Tian, A. M. Chin. J. Org. Chem. 2013, 33, 2169. (in Chinese)
doi: 10.6023/cjoc201303011 |
|
( 张明, 王玲玲, 李来才, 田安明, 有机化学, 2013, 33, 2169.)
doi: 10.6023/cjoc201303011 |
|
| [9] |
Bai, D. H.; Li, C. L.; Li, J.; Jia, X. S. Chin. J. Org. Chem. 2012, 32, 994. (in Chinese)
doi: 10.6023/cjoc1202073 |
|
( 白东虎, 李春举, 李健, 贾学顺, 有机化学, 2012, 32, 994.)
doi: 10.6023/cjoc1202073 |
|
| [10] |
Hay, A. S. J. Org. Chem. 1962, 27, 3320.
doi: 10.1021/jo01056a511 |
| [11] |
Evano, G.; Blanchard, N. Copper-Mediated Cross-Coupling Reactions, John Wiley & Sons, Inc., Hoboken, New Jersey, Published simultaneously in Canada, 2013.
|
| [12] |
Karlin, K. D.; Itoh, S. Copper-Oxygen Chemistry, John Wiley & Sons, Inc., Hoboken, New Jersey, Published simultaneously in Canada, 2011.
|
| [13] |
Su, L. B.; Dong, J. Y.; Liu, L.; Sun, M. L.; Qiu, R. H.; Zhou, Y. B.; Yin, S. F. J. Am. Chem. Soc. 2016, 138, 12348.
doi: 10.1021/jacs.6b07984 |
| [14] |
Zhang, Z. H.; Dong, X. Y.; Du, X. Y.; Gu, Q. S.; Li, Z. L.; Liu, X. Y. Nat. Commun. 2019, 10, 5689.
doi: 10.1038/s41467-019-13705-1 |
| [15] |
Siemsen, P.; Livingston, R. C.; Diederich, F. Angew. Chem., Int. Ed. 2000, 39, 2632.
doi: 10.1002/1521-3773(20000804)39:15【-逻*辑*与-】#x00026;lt;2632::AID-ANIE2632【-逻*辑*与-】#x00026;gt;3.0.CO;2-F |
| [16] |
Bohlmann, F.; Schnowsky, H.; Inhoffen, E.; Grau, G.; Chem. Ber. 1964, 97, 794.
doi: 10.1002/cber.19640970322 |
| [17] |
Seavill, P. W.; Holt, K. B.; Wilden, J. D. Faraday Discuss. 2019, 220, 269.
doi: 10.1039/c9fd00031c pmid: 31502612 |
| [18] |
Jiang, H. F.; Tang, J. Y.; Wang, A. Z.; Deng, G. H.; Yang, S. R. Synthesis 2006, 7, 1155.
|
| [19] |
Fomina, L.; Vazquez, B.; Tkatchouk, E.; Fomine, S. Tetrahedron 2002, 58, 6741.
doi: 10.1016/S0040-4020(02)00669-5 |
| [20] |
Bakhoda, A.; Okoromoba, O. E.; Greene, C.; Boroujeni, M. R.; Bertke, J. A.; Warren, T. H. J. Am. Chem. Soc. 2020, 142, 18483.
doi: 10.1021/jacs.0c07137 |
| [21] |
Bai, R. P.; Zhang, G. G.; Yi, H.; Huang, Z. L.; Qi, X.T.; Liu, C.; Miller, J. T.; Kropf, A. J.; Bunel, E. E.; Lan, Y.; Lei, A.W. J. Am. Chem. Soc. 2014, 136, 16760.
doi: 10.1021/ja5097489 |
| [22] |
Houmam, A. Chem. Rev. 2008, 108, 2180.
|
| [23] |
Wu, L. M.; Guo, X.; Wang, J.; Guo, Q. X.; Liu, Z. L.; Liu, Y. C. Sci. China, Ser. B Chem. 1998, 27, 540. (in Chinese)
|
|
( 吴隆民, 郭霞, 王隽, 郭庆祥, 刘中立, 刘有成, 中国科学(B辑), 1998, 27, 540.)
|
|
| [24] |
Luo, S.; Wei, Z. S.; Dionysiou, D. D.; Spinney, R.; Hu, W. P.; Chai, L. Y.; Ye, T. T.; Xiao, R. Y. Chem. Eng. J. 2017, 327, 1056.
doi: 10.1016/j.cej.2017.06.179 |
| [25] |
Wang, D.; Huang, S. P.; Wang, C.; Yue, Y.; Zhang, Q. S. Org. Electron. 2019, 64, 216.
doi: 10.1016/j.orgel.2018.10.038 |
| [26] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L.; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr, J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 09, Rev. B.01, Wallingford, CT, 2010.
|
| [27] |
Wang, G.; Bai, K. K.; Li, L. C.; Tian, A. M. Sci. China-Chem. 2014, 44, 334. (in Chinese)
|
|
( 王刚, 白坤坤, 李来才, 田安民, 中国科学:化学, 2014, 44, 334.)
|
|
| [28] |
Zhao, Y.; Truhlar, D. G. Theor. Chem. Acc. 2008, 120, 215.
doi: 10.1007/s00214-007-0310-x |
| [29] |
Wu, T. Z.; Kalugina, Y. N.; Thakkar, A. J. Chem. Phys. Lett. 2015, 635, 257.
doi: 10.1016/j.cplett.2015.07.003 |
| [30] |
Alecu, I. M.; Zheng, J. J.; Zhao, Y.; Truhlar, D. G. J. Chem. Theory Comput. 2010, 6, 2872.
doi: 10.1021/ct100326h pmid: 26616087 |
| [31] |
Man, Q. M.; Fu, Z. Y.; Liu, T. T.; Zheng, M. Y.; Jiang, H. L. Acta Chim. Sinica 2021, 79, 948. (in Chinese)
doi: 10.6023/A21040172 |
|
( 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良, 化学学报 2021, 79, 948.)
doi: 10.6023/A21040172 |
|
| [32] |
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
doi: 10.1021/jp810292n |
| [33] |
Gao, Y. P.; Ji, Y. M.; Li, G. Y.; An, T. C. Water Res. 2014, 493, 60.
|
| [34] |
Luo, S.; Wei, Z. S.; Spinney, R.; Villamena, F. A.; Dionysiou, D. D.; Cheng, D.; Tang, C. J.; Chai, L. Y.; Xiao, R. Y. J. Hazard. Mater. 2018, 344, 1165.
doi: 10.1016/j.jhazmat.2017.09.024 |
| [35] |
Xiao, R.; Noerpel, M.; Luk, H.L.; Wei, Z.S.; Spinney, R. Int. J. Quantum Chem. 2014, 114, 74.
doi: 10.1002/qua.24518 |
| [36] |
Nelsen, S. F.; Weaver, M. N.; Luo, Y.; Pladziewicz, J. R.; Ausman, L. K.; Jentzsch, T. L; O’Konek, J. J. J. Phys. Chem. 2012, 110, 11665.
|
| [37] |
Ziegler, M.S.; Lakshmi, K.V.; Tilley, T. D. J. Am. Chem. Soc. 2017, 139, 5378.
doi: 10.1021/jacs.6b13261 pmid: 28394586 |
| [38] |
Kundu, S.; Greene, C.; Williams, K. D.; Salvador, T. K.; Bertke, J. A.; Cundari, T. R.; Warren, T. H. J. Am. Chem. Soc. 2017, 139, 9112.
doi: 10.1021/jacs.7b04046 |
| [1] | Wei Xu, Hongbin Zhai, Bin Cheng, Taimin Wang. Visible Light-Induced Pd-Catalyzed Heck Reactions [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3035-3054. |
| [2] | Xiaona Yang, Hongyu Guo, Rong Zhou. Progress in Visible-Light Promoted Transformations of Organosilicon Compounds [J]. Chinese Journal of Organic Chemistry, 2023, 43(8): 2720-2742. |
| [3] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [4] | Ju Peng, Xiaoqian He, Li-Li Liao, Ruopeng Bai, Yu Lan. Theoretical Study of How Electronic Effect of Substituent Affects Regioselectivity of C—Si Reductive Elimination [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3608-3613. |
| [5] | Lin Ling, Jian Wang, Jing Li, Yuxue Li, Long Lu. Broken-Symmetry Density Functional Theory Study on Pyrolysis Mechanisms of 3-Nitro-1,2,4-triazol-5-one (NTO) [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 285-294. |
| [6] | Geyang Song, Dong Xue. Research Progress on Light-Promoted Transition Metal-Catalyzed C-Heteroatom Bond Coupling Reactions [J]. Chinese Journal of Organic Chemistry, 2022, 42(8): 2275-2299. |
| [7] | Yubing Shi, Wenji Bai, Weihua Mu, Jiangping Li, Jiawei Yu, Bing Lian. Research Progress on Density Functional Theory Study of Palladium-Catalyzed C—H Functionalization to Form C—X (X=O, N, F, I, …) Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1346-1374. |
| [8] | Weiguo Yu, Lingna Wang, Xiaocong Yu, Shuping Luo. Fluorescent Dye/Nickel Synergistic Catalytic Decarboxylative Carbonylation Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 1216-1223. |
| [9] | Zheng Li, Yingchun Gu, Dazhen Xu, Xuening Fei, Lei Zhang. Density Functional Theory Study on the Mechanism of Organophosphine-Catalyzed [4+2] Cycloaddition Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 830-837. |
| [10] | Man Xu, Yuanzhi Xia. Mechanistic Understanding of Rh(III)-Catalyzed Redox-Neutral C—H Activation/Annulation Reactions of N-Phenoxyacetamides and Methyleneoxetanones [J]. Chinese Journal of Organic Chemistry, 2021, 41(8): 3272-3278. |
| [11] | Li Huang, Yuhao Wang, Jiying Liu, Shijun Li, Wenjing Zhang, Yu Lan. Mechanistic Study of Cu-Catalyzed Addition Reaction of lsocyanates [J]. Chinese Journal of Organic Chemistry, 2021, 41(11): 4347-4352. |
| [12] | Cao Shanshan, Liu Zhaohong, Yuan Haiyan, Yang Liu, Zhang Jingping, Bi Xihe. Computational Studies on Reaction Mechanism of the Catalyst-Controlled Selective Insertion of Metal Carbenoids into C-C and C-H Bonds of 1,3-Dicarbonyl Compounds [J]. Chinese Journal of Organic Chemistry, 2020, 40(8): 2468-2475. |
| [13] | Shi Dunfa, Wang Lu, Xia Chungu, Liu Chao. Recent Advances in Visible-Light-Promoted Transformation of Alkyl Boron Compounds [J]. Chinese Journal of Organic Chemistry, 2020, 40(11): 3605-3619. |
| [14] | Li Yingjun, Zhao Yue, Jin Kun, Gao Lixin, Sheng Li, Liu Xuejie, Yang Hongjing, Lin Ledi, Li Jia. Synthesis and PTP1B/TCPTP Inhibitory Activity Evaluation of Novel 2,5-Disubstituted-1,3,4-thiadiazolamide Derivatives Containing Carbazole/Benzimidazole Moity [J]. Chin. J. Org. Chem., 2019, 39(9): 2599-2608. |
| [15] | Wang Wanjun, Li Huan, Pan Renming, Zhu Weihua. Molecular Design of High Energy Density Materials with Bis(3,4,5-substituted-pyrazolyl)methane Derivatives [J]. Chin. J. Org. Chem., 2019, 39(5): 1362-1371. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||