Chinese Journal of Organic Chemistry ›› 2022, Vol. 42 ›› Issue (3): 830-837.DOI: 10.6023/cjoc202109022 Previous Articles Next Articles
ARTICLES
李征a, 谷迎春a,*( ), 徐大振b, 费学宁a, 张磊a,*(
), 徐大振b, 费学宁a, 张磊a,*( )
)
收稿日期:2021-09-16
修回日期:2021-10-27
发布日期:2021-11-17
通讯作者:
谷迎春, 张磊
基金资助:
Zheng Lia, Yingchun Gua( ), Dazhen Xub, Xuening Feia, Lei Zhanga(
), Dazhen Xub, Xuening Feia, Lei Zhanga( )
)
Received:2021-09-16
Revised:2021-10-27
Published:2021-11-17
Contact:
Yingchun Gu, Lei Zhang
Supported by:Share
Zheng Li, Yingchun Gu, Dazhen Xu, Xuening Fei, Lei Zhang. Density Functional Theory Study on the Mechanism of Organophosphine-Catalyzed [4+2] Cycloaddition Reaction[J]. Chinese Journal of Organic Chemistry, 2022, 42(3): 830-837.
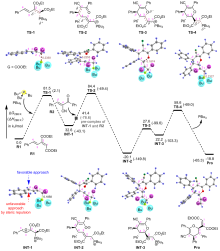
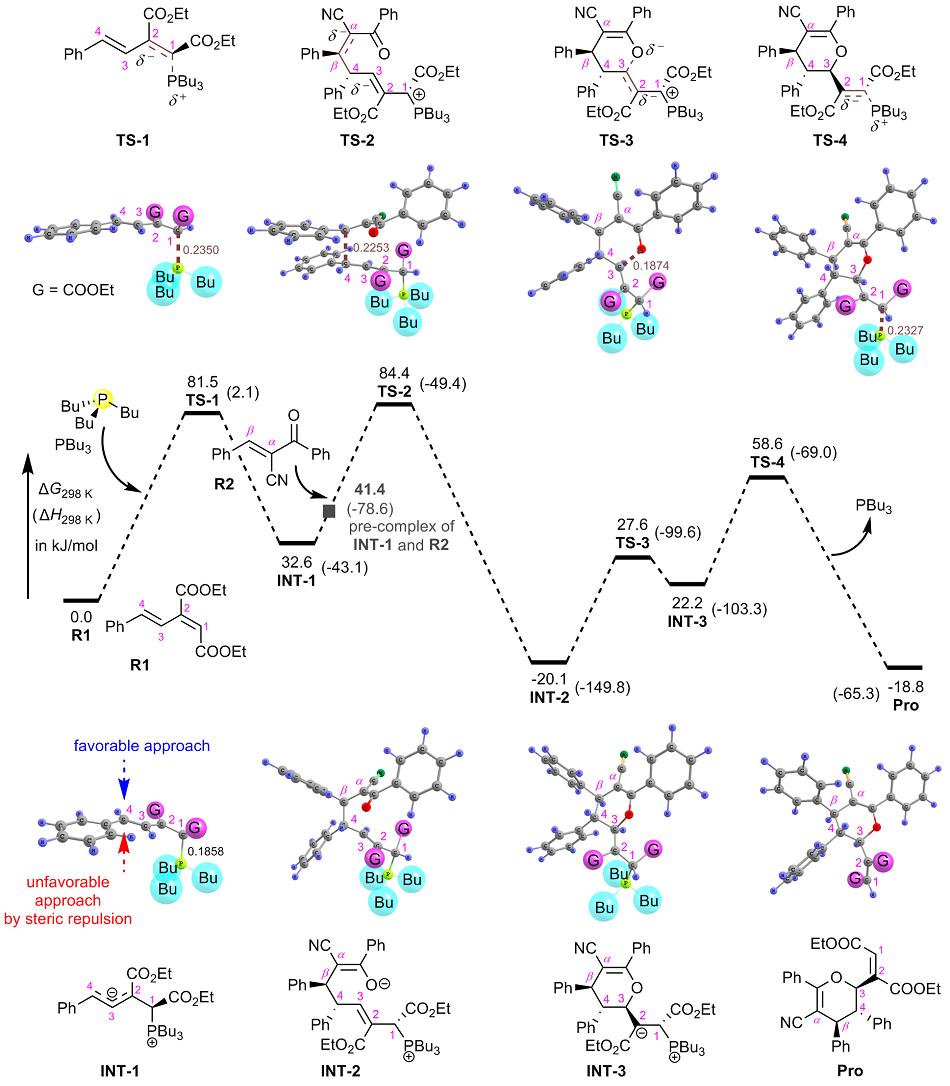
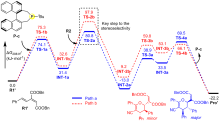


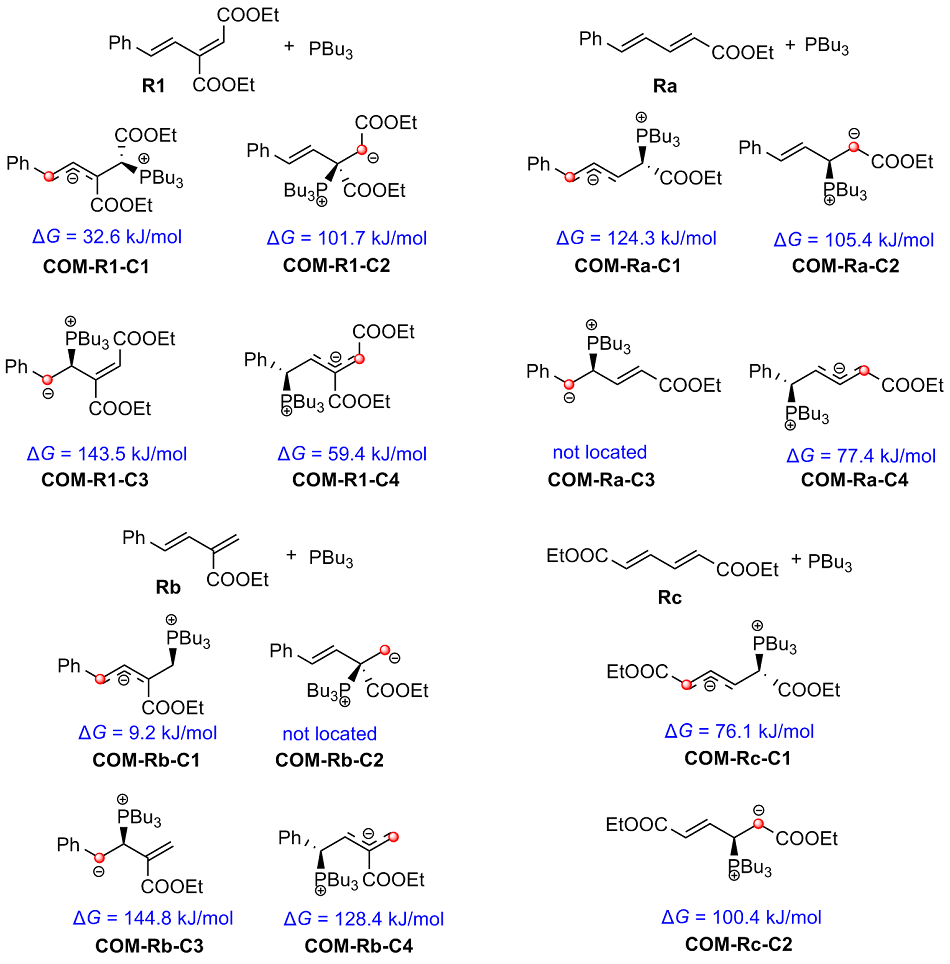
| [1] |
(a) Wang, N.-Z.; Wu, Z.-G.; Wang, J.-J.; Ullah, N.; Lu, Y.-X. Chem. Soc. Rev. 2021, 50, 9766.
doi: 10.1039/D0CS01124J |
|
(b) Cai, W.; Huang, Y. Chin. J. Org. Chem. 2021, 41, 3903. (in Chinese)
doi: 10.6023/cjoc202106004 |
|
|
(蔡卫, 黄有, 有机化学, 2021, 41, 3903.)
|
|
| [2] |
(a) Huang, Y.-F.; Liao, J.-N.; Wang, W. Chem. Commun. 2020, 56, 15235.
doi: 10.1039/D0CC05699E |
|
(b) Ni, H.-Z.; Chan, W.-L.; Lu, Y.-X. Chem. Rev. 2018, 118, 9344.
doi: 10.1021/acs.chemrev.8b00261 |
|
| [3] |
(a) Wang, H.-M.; Zhou, W.; Tao, M.-N.; Hu, A.-J.; Zhang, J.-L. Org. Lett. 2017, 19, 1710.
doi: 10.1021/acs.orglett.7b00489 |
|
(b) Tang, X.-D.; Tan, C.-X. A.; Chan, W.-L.; Zhang, F.-H.; Zheng, W.-R.; Lu, Y.-X. ACS Catal. 2021, 11, 1361.
doi: 10.1021/acscatal.0c05225 |
|
| [4] |
Ni, H.-Z.; Wong, Y.-L.; Wu, M.-Y.; Han, Z.-B.; Ding, K.-L.; Lu, Y.-X. Org. Lett. 2020, 22, 2460.
doi: 10.1021/acs.orglett.0c00681 |
| [5] |
(a) Ziegler, D.-T.; Riesgo, L.; Ikeda, T.; Fujiwara, Y.; Fu, G.-C. Angew. Chem., Int. Ed. 2014, 53, 13183.
doi: 10.1002/anie.201405854 |
|
(b) Yang, M.; Cao, S.-X.; He, Z.-J. Chin. J. Org. Chem. 2019, 39, 2235. (in Chinese)
doi: 10.6023/cjoc201904041 |
|
|
(杨梅, 曹仕轩, 贺峥杰, 有机化学, 2019, 39, 2235.)
doi: 10.6023/cjoc201904041 |
|
| [6] |
(a) Tran, Y.-S.; Kwon, O. J. Am. Chem. Soc. 2007, 129, 12632.
doi: 10.1021/ja0752181 |
|
(b) Gu, Y.-C.; Huang, Y. Chin. J. Org. Chem. 2019, 39, 2251. (in Chinese)
|
|
|
(谷迎春, 黄有, 有机化学, 2019, 39, 2251.)
doi: 10.6023/cjoc201904017 |
|
| [7] |
Huang, G.-F.; Ren, X.-Y.; Jiang, C.-H.; Wu, H.-J.; Gao, G.-W.; Wang, T.-L. Org. Chem. Front. 2019, 6, 2872.
doi: 10.1039/C9QO00478E |
| [8] |
(a) Jin, Z.-X.; Ni, H.-Z.; Zhou, B.; Zheng, W.-R.; Lu, Y.-X. Org. Lett. 2018, 20, 5515.
doi: 10.1021/acs.orglett.8b02519 |
|
(b) Zhang, X.-N.; Deng, H.-P.; Huang, L.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 8664.
doi: 10.1039/c2cc34619b |
|
| [9] |
(a) Schuler, M.; Duvvuru, D.; Retailleau, P.; Betzer, J.-F.; Marinett, A. Org. Lett. 2009, 11, 4406.
doi: 10.1021/ol901758k pmid: 19725549 |
|
(b) Li, N.; Jia, P.-H.; Huang, Y. Chem. Commun. 2019, 55, 10976.
doi: 10.1039/C9CC05832J pmid: 19725549 |
|
| [10] |
(a) He, X.; Tang, Y.-H.; Wang, Y.-Z.; Chen, J.-B.; Xu, S.-L.; Dou, J.-W.; Li, Y. Angew. Chem., Int. Ed. 2019, 58, 10698.
doi: 10.1002/anie.v58.31 |
|
(b) Wu, J.; Tang, Y.-H.; Wei, W.; Wu, Y.; Li, Y.; Zhang, J.-J.; Zheng, Y.-S.; Xu, S.-L. Angew. Chem., Int. Ed. 2018, 57, 6284.
doi: 10.1002/anie.v57.21 |
|
| [11] |
(a) Zhang, K.; Wang, Y.; Zhu, H.; Peng, Q. Chin. J. Org. Chem. 2021, 41, 3995. (in Chinese)
doi: 10.6023/cjoc202102036 |
|
(张凯瑞, 王亚亚, 朱宏丹, 彭谦, 有机化学, 2021, 41, 3995.)
|
|
|
(b) Li, S.-J.; Fang, D.-C. Phys. Chem. Chem. Phys. 2016, 18, 30815.
doi: 10.1039/C6CP05190A |
|
| [12] |
(a) Huang, G.-T.; Lankau, T.; Yu, C.-H. J. Org. Chem. 2014, 79, 1700.
doi: 10.1021/jo402609v |
|
(b) Yu, Z.-Y.; Jin, Z.-J.; Duan, M.; Bai, R.-P.; Lu, Y.-X.; Lan, Y. J. Org. Chem. 2018, 83, 9729.
doi: 10.1021/acs.joc.8b01259 |
|
|
(c) Wu, Y.; Li, M.-Z.; Jin, L.; Zhao, X. ACS Omega 2020, 5, 2957.
doi: 10.1021/acsomega.9b03902 |
|
|
(d) Li, Y.; Zhang, Z.-Q. New J. Chem. 2019, 43, 13600.
doi: 10.1039/C9NJ01943J |
|
| [13] |
Li, K.; Gan, Z.-J.; Li, E.-Q.; Duan, Z. Org. Lett. 2021, 23, 3094.
doi: 10.1021/acs.orglett.1c00780 |
| [14] |
Lu, T.; Chen, F. J. Comput. Chem. 2012, 33, 580.
doi: 10.1002/jcc.v33.5 |
| [15] |
Johnson, E. R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A. J.; Yang, W. J. Am. Chem. Soc. 2010, 132, 6498.
doi: 10.1021/ja100936w pmid: 20394428 |
| [16] |
Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. A.; Nakatsuji, H.; Caricato, M.; Li, X.; Hratchian, H. P.; Izmaylov, A. F.; Bloino, J.; Zheng, G.; Sonnenberg, J. L.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Montgomery, J. A., Jr.; Peralta, J. E.; Ogliaro, F.; Bearpark, M.; Heyd, J. J.; Brothers, E.; Kudin, K. N.; Staroverov, V. N.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Rega, N.; Millam, J. M.; Klene, M.; Knox, J. E.; Cross, J. B.; Bakken, V.; Adamo, C.; Jaramillo, J.; Gomperts, R.; Stratmann, R. E.; Yazyev, O.; Austin, A. J.; Cammi, R.; Pomelli, C.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Zakrzewski, V. G.; Voth, G. A.; Salvador, P.; Dannenberg, J. J.; Dapprich, S.; Daniels, A. D.; Farkas, O.; Foresman, J. B.; Ortiz, J. V.; Cioslowski, J.; Fox, D. J. Gaussian 09, Revision D.01, Gaussian, Inc., Wallingford, CT, 2013.
|
| [17] |
Zhao, Y.; Truhlar, D. G. J. Chem. Phys. 2006, 125, 194101.
doi: 10.1063/1.2370993 |
| [18] |
Scalmani, G.; Frisch, M. J. J. Chem. Phys. 2010, 132, 114110.
doi: 10.1063/1.3359469 |
| [19] |
Fukui, K. Acc. Chem. Res. 1981, 14, 363.
doi: 10.1021/ar00072a001 |
| [20] |
Marenich, A. V.; Cramer, C. J.; Truhlar, D. G. J. Phys. Chem. B 2009, 113, 6378.
doi: 10.1021/jp810292n |
| [21] |
(a) Mammen, M.; Shakhnovich, E. I.; Deutch, J. M.; Whitesides, G. M. J. Org. Chem. 1998, 63, 3821.
doi: 10.1021/jo970944f pmid: 25714789 |
|
(b) Besora, M.; Vidossich, P.; Lledós, A.; Ujaque, G.; Maseras, F. J. Phys. Chem. A 2018, 122, 1392.
doi: 10.1021/acs.jpca.7b11580 pmid: 25714789 |
|
|
(c) Liang, Y.; Liu, S.; Xia, Y.; Li, Y.; Yu, Z.-X. Chem.-Eur. J. 2008, 14, 4361.
doi: 10.1002/chem.200701725 pmid: 25714789 |
|
|
(d) Plata, R. E.; Singleton, D. A. J. Am. Chem. Soc. 2015, 137, 3811.
doi: 10.1021/ja5111392 pmid: 25714789 |
|
|
(e) Han, L.-L.; Li, S.-J.; Fang, D.-C. Phys. Chem. Chem. Phys. 2016, 18, 6182
doi: 10.1039/C5CP07803B pmid: 25714789 |
|
| [22] |
(a) Huang, F.; Lu, G.; Zhao, L.; Li, H.; Wang, Z.-X. J. Am. Chem. Soc. 2010, 132, 12388.
doi: 10.1021/ja103531z pmid: 20707349 |
|
(b) Li, H.; Lu, G.; Jiang, J.; Huang, F.; Wang, Z.-X. Organometallics 2011, 30, 2349.
doi: 10.1021/om200089m pmid: 20707349 |
|
|
(c) Zhang, L.; Jiang, B.; Chen, Y.; Lü, J.-F.; Feng, W.-C. Eur. J. Org. Chem. 2019, 6217.
pmid: 20707349 |
| [1] | Jingrui Wang, Yongkui Feng, Nengzhong Wang, Nianyu Huang, Hui Yao. Pd-Catalyzed Stereoselective Synthesis of Nitroalkyl β-C-Glycosides [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3216-3225. |
| [2] | Huanqing Li, Zhaohua Chen, Zujia Chen, Qiwen Qiu, Youcai Zhang, Sihong Chen, Zhaoyang Wang. Research Progress in Mercury Ion Fluorescence Probes Based on Organic Small Molecules [J]. Chinese Journal of Organic Chemistry, 2023, 43(9): 3067-3077. |
| [3] | Cunjing Miao, Jiaqi Yao. Recent Advances in the Transformation Reactions of Aromatic Nitriles via C—CN Bond Cleavage [J]. Chinese Journal of Organic Chemistry, 2023, 43(4): 1341-1364. |
| [4] | Xun Xiang, Zhaolin He, Xiuqin Dong. Recent Advances of Efficient Synthesis of Chiral Molecules Promoted by Pd/Chiral Phosphoric Acid Synergistic Catalysis [J]. Chinese Journal of Organic Chemistry, 2023, 43(3): 791-808. |
| [5] | Tingting Liu, Yucai Hu, An Shen. Mechanism of Carbon-Carbon Coupling Reactions Catalyzed by Imine-Ligand-Assisted N-Heterocyclic Carbene Palladium Complexes [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 622-628. |
| [6] | Yueling Liu, Xinxin Zhong, Ganbing Zhang. Density Functional Theory Study for Exploring the Mechanisms of the [3+2] Cycloaddition Reactions between 1-R-3-Phenylpropylidenecyclopropane (R=Me/H) and Furfural Catalyzed by Pd(0) [J]. Chinese Journal of Organic Chemistry, 2023, 43(2): 660-667. |
| [7] | Kang Pan, Fan Xu. Lanthanum Silylamide-Catalyzed Synthesis of Enol Phosphates [J]. Chinese Journal of Organic Chemistry, 2023, 43(12): 4261-4267. |
| [8] | Ju Peng, Xiaoqian He, Li-Li Liao, Ruopeng Bai, Yu Lan. Theoretical Study of How Electronic Effect of Substituent Affects Regioselectivity of C—Si Reductive Elimination [J]. Chinese Journal of Organic Chemistry, 2023, 43(10): 3608-3613. |
| [9] | Lin Ling, Jian Wang, Jing Li, Yuxue Li, Long Lu. Broken-Symmetry Density Functional Theory Study on Pyrolysis Mechanisms of 3-Nitro-1,2,4-triazol-5-one (NTO) [J]. Chinese Journal of Organic Chemistry, 2023, 43(1): 285-294. |
| [10] | Zexin Huang, Yuqiang Yin, Fengcheng Jia, Anxin Wu. Research Progress on C2—C3 Bond Cleavage of Indole and Its Derivatives [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2028-2044. |
| [11] | Xueli Zhu, Shaoli Yang, Caixia Jia, Jing Li, Zheng Duan. Synthesis of Dihydrobenzooxaphospholes via Cyclocondensation of 2-Phosphinylphenol and Aldehydes [J]. Chinese Journal of Organic Chemistry, 2022, 42(7): 2201-2213. |
| [12] | Mengnan Lai, Qiuyuan Wang, Min Hua, Nianyu Huang, Hui Yao. Open-Air Stereoselective Synthesis of C-Aryl Fucosides/Arabinosides [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1694-1705. |
| [13] | Liwen Ma, Xiaoye Wei, Zilin Zhao, Ang Zhao, Xiangwen Deng, Bingnan Huo, Gang Ma, Chunfang Zhang. Theoretical Study on the Catalytic Mechanism of Copper with Various Valence for the Terminal Alkyne Coupling Reaction [J]. Chinese Journal of Organic Chemistry, 2022, 42(6): 1811-1819. |
| [14] | Yubing Shi, Wenji Bai, Weihua Mu, Jiangping Li, Jiawei Yu, Bing Lian. Research Progress on Density Functional Theory Study of Palladium-Catalyzed C—H Functionalization to Form C—X (X=O, N, F, I, …) Bonds [J]. Chinese Journal of Organic Chemistry, 2022, 42(5): 1346-1374. |
| [15] | Youcai Zhu, Xinxin Ding, Li Sun, Zhen Liu. Advances in the Production of Acrylic Acid and Its Derivatives by CO2/C2H4 Coupling [J]. Chinese Journal of Organic Chemistry, 2022, 42(4): 965-977. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||