化学学报 ›› 2023, Vol. 81 ›› Issue (5): 456-460.DOI: 10.6023/A23020048 上一篇 下一篇
所属专题: 庆祝《化学学报》创刊90周年合辑
研究论文
投稿日期:2023-02-24
发布日期:2023-05-22
作者简介:基金资助:
Fangyan Yuan, Chao Li, Meiming Luo( ), Xiaoming Zeng(
), Xiaoming Zeng( )
)
Received:2023-02-24
Published:2023-05-22
Contact:
*E-mail: luomm@scu.edu.cn; zengxiaoming@scu.edu.cn
About author:Supported by:文章分享
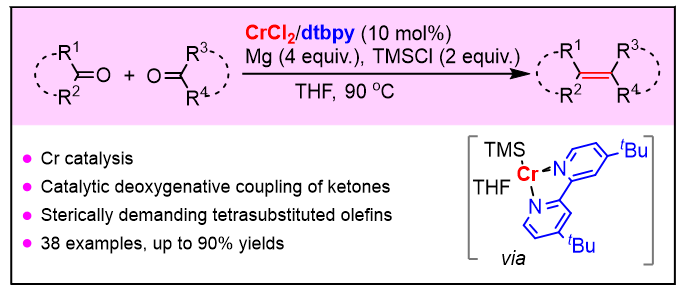
四取代烯烃是重要的基础化学品, 被广泛应用于材料、药物化学等领域. 发展新型、高效的催化合成策略制备四取代烯烃具有重要的研究意义. 本工作以商业易得的酮类化合物为原料、廉价的二氯化铬为催化剂前体、4,4'-二叔丁基-2,2'-联吡啶作为配体、镁为还原剂, 在三甲基氯硅烷的作用下, 实现了酮羰基的脱氧自偶联和交叉偶联反应, 一步合成了空间位阻较大的四取代烯烃. 机理研究表明, 原位生成的低价铬有可能与镁及三甲基氯硅烷作用形成硅基铬物种, 插入酮羰基形成硅氧基取代的烷基铬中间体; 紧接着与另一分子酮羰基发生加成和脱氧反应生成四取代烯烃.
袁芳艳, 李超, 罗美明, 曾小明. 铬催化酮羰基的脱氧偶联反应合成四取代烯烃★[J]. 化学学报, 2023, 81(5): 456-460.
Fangyan Yuan, Chao Li, Meiming Luo, Xiaoming Zeng. Chromium-Catalyzed Carbonyl-Carbonyl Deoxygenative Couplings of Ketones to Tetrasubstituted Olefins★[J]. Acta Chimica Sinica, 2023, 81(5): 456-460.
| |
| |
| [1] |
(a) Duan, X.-F.; Zeng, J.; Lü, J.-W.; Zhang, Z.-B. J. Org. Chem. 2006, 71, 9873.
doi: 10.1021/jo061644d pmid: 25686761 |
|
(b) Duan, X.-F.; Zeng, J.; Lü, J.-W.; Zhang, Z.-B. Synthesis 2007, 5, 713.
pmid: 25686761 |
|
|
(c) Flynn, A. B.; Ogilvie, W. W. Chem. Rev. 2007, 107, 4698.
doi: 10.1021/cr050051k pmid: 25686761 |
|
|
(d) Liang, J.; Tang, B. Z.; Liu, B. Chem. Soc. Rev. 2015, 44, 2798.
doi: 10.1039/c4cs00444b pmid: 25686761 |
|
|
(e) Qi, Y.; Wang, Y.; Yu, Y.; Liu, Z.; Zhang, Y.; Zhou, C. J. Mater. Chem. C 2016, 4, 11291.
doi: 10.1039/C6TC04215E pmid: 25686761 |
|
|
(f) Zhang, G.-F.; Wang, H.; Aldred, M. P.; Chen, T.; Chen, Z.-Q.; Meng, X.; Zhu, M.-Q. Chem. Mater. 2014, 26, 4433.
doi: 10.1021/cm501414b pmid: 25686761 |
|
| [2] |
(a) Kahn, B. E.; Rieke, R. D. Chem. Rev. 1988, 88, 733.
doi: 10.1021/cr00087a002 |
|
(b) McMurry, J. E. Chem. Rev. 1989, 89, 1513.
doi: 10.1021/cr00097a007 |
|
|
(c) McMurry, J. E.; Lectka, T.; Rico, J. G. J. Org. Chem. 1989, 54, 3748.
doi: 10.1021/jo00276a047 |
|
|
(d) Balu, N.; Nayak, S. K.; Banerji, A. J. Am. Chem. Soc. 1996, 118, 5932.
doi: 10.1021/ja9535221 |
|
|
(e) Takeda, T. Modern Carbonyl Olefination: Methods and Applications, Wiley-VCH, Weinheim, 2004.
|
|
|
(f) Duan, Z.-Y.; Zhang, J.-Z.; Xu, X.-X. Acta Chim. Sinica 2004, 62, 811. (in Chinese)
|
|
|
(段志勇, 张家仲, 许杏祥, 化学学报, 2004, 62, 811.)
|
|
|
(g) Wang, Z.; Xiao, Y.; Jin, H.; Tan, T.; Wang, S.; Li, X. Acta Chim. Sinica 2014, 72, 731. (in Chinese)
doi: 10.6023/A14030158 |
|
|
(王志强, 肖殷, 金会义, 谈廷风, 王世荣, 李祥高, 化学学报, 2014, 72, 731.)
doi: 10.6023/A14030158 |
|
| [3] |
(a) Fürstner, A.; Bogdanović, B. Angew. Chem. Int. Ed. Engl. 1996, 35, 2442.
doi: 10.1002/(ISSN)1521-3773 |
|
(b) Ephritikhine, M. Chem. Commun. 1998, 2549.
|
|
|
(c) Hertz, V. M.; Bolte, M.; Lerner, H.-W.; Wagner, M. Angew. Chem. Int. Ed. 2015, 54, 8800.
doi: 10.1002/anie.201502977 |
|
| [4] |
(a) Diéguez, H. R.; López, A.; Domingo, V.; Arteaga, J. F.; Dobado, J. A.; Herrador, M. M.; Moral, J. F. Q.; Barrero, A. F. J. Am. Chem. Soc. 2010, 132, 254.
doi: 10.1021/ja906083c |
|
(b) Zhang, L.; Yu, X.; Zhang, L.; Zhou, X.; Lin, Y. Org. Chem. Front. 2014, 1, 929.
doi: 10.1039/C4QO00140K |
|
|
(c) Fürstner, A.; Hupperts, A. J. Am. Chem. Soc. 1995, 117, 4468.
doi: 10.1021/ja00121a004 |
|
| [5] |
Xia, Y.; Liu, Z.; Xiao, Q.; Qu, P.; Ge, R.; Zhang, Y.; Wang, J. Angew. Chem. Int. Ed. 2012, 51, 5714.
doi: 10.1002/anie.201201374 |
| [6] |
Wei, W.; Dai, X.-J.; Wang, H.; Li, C.; Yang, X.; Li, C.-J. Chem. Sci. 2017, 8, 8193.
doi: 10.1039/c7sc04207h pmid: 29568466 |
| [7] |
Wang, S.; Lokesh, N.; Hioe, J.; Gschwind, R. M.; König, B. Chem. Sci. 2019, 10, 4580.
doi: 10.1039/C9SC00711C |
| [8] |
Banerjee, S.; Kobayashi, T.; Takai, K.; Asako, S.; Ilies, L. Org. Lett. 2022, 24, 7242.
doi: 10.1021/acs.orglett.2c03143 |
| [9] |
Liu, C.-F.; Wang, H.; Martin, R. T.; Zhao, H.; Gutierrez, O.; Koh, M. J. Nat. Catal. 2021, 4, 674.
doi: 10.1038/s41929-021-00658-2 |
| [10] |
Baati, R.; Mioskowski, C.; Barma, D.; Kache, R.; Falck, J. R. Org. Lett. 2006, 8, 2949.
doi: 10.1021/ol0607140 |
| [11] |
(a) Cong, X.; Zeng, X. Acc. Chem. Res. 2021, 54, 2014.
doi: 10.1021/acs.accounts.1c00096 |
|
(b) Zeng, X.; Cong, X. Org. Chem. Front. 2015, 2, 69.
doi: 10.1039/C4QO00272E |
|
|
(c) Li, J.; Knochel, P. Synthesis 2019, 51, 2100.
doi: 10.1055/s-0037-1611756 |
|
|
(d) Ye, Y.; Gong, H. Chin. J. Org. Chem. 2020, 40, 2588. (in Chinese)
doi: 10.6023/cjoc202000048 |
|
|
(叶杨, 龚和贵, 有机化学, 2020, 40, 2588.)
doi: 10.6023/cjoc202000048 |
|
| [12] |
(a) Cong, X.; Tang, H.; Zeng, X. J. Am. Chem. Soc. 2015, 137, 14367.
doi: 10.1021/jacs.5b08621 pmid: 31453706 |
|
(b) Tang, J.; Liu, L. L.; Yang, S.; Cong, X.; Luo, M.; Zeng, X. C J. Am. Chem. Soc. 2020, 142, 7715.
doi: 10.1021/jacs.0c00283 pmid: 31453706 |
|
|
(c) Rong, Z.; Luo, M.; Zeng, X. Org. Lett. 2019, 21, 6869.
doi: 10.1021/acs.orglett.9b02504 pmid: 31453706 |
|
|
(d) Ling, L.; Cheng, C.; Luo, M.; Zeng, X. Org. Lett. 2019, 21, 1912.
doi: 10.1021/acs.orglett.9b00554 pmid: 31453706 |
|
|
(e) Yin, J.; Li, J.; Wang, G.-X.; Yin, Z.-B.; Zhang, W.-X.; Xi, Z. J. Am. Chem. Soc. 2019, 141, 4241.
doi: 10.1021/jacs.9b00822 pmid: 31453706 |
|
|
(f) Zhang, Y.; Zhu, S. Chin. J. Org. Chem. 2021, 41, 1255. (in Chinese)
doi: 10.6023/cjoc202100017 pmid: 31453706 |
|
|
(张艳东, 朱守非, 有机化学, 2021, 41, 1255.)
doi: 10.6023/cjoc202100017 pmid: 31453706 |
|
| [13] |
Navale, T. S.; Thakur, K.; Rathore, R. Org. Lett. 2011, 13, 1634.
doi: 10.1021/ol200069c |
| [14] |
Zhao, L.; Hu, C.; Cong, X.; Deng, G.; Liu, L. L.; Luo, M.; Zeng, X. J. Am. Chem. Soc. 2021, 143, 1618.
doi: 10.1021/jacs.0c12318 |
| [15] |
Mock, M. T.; Chen, S.; O’Hagan, M.; Rousseau, R.; Dougherty, W. G.; Kassel, W. S.; Bullock, R. M. J. Am. Chem. Soc. 2013, 135, 11493.
doi: 10.1021/ja405668u |
| [1] | 董文锋, 王楠, 杨贺, 徐广庆, 汤文军. 聚合型膦配体在除草剂灵思科中的合成应用[J]. 化学学报, 2024, 82(9): 940-953. |
| [2] | 卡迪尔亚•阿布都外力, 热孜古丽•玉努斯, 李佳佳, 罗时玮, 阿布都热西提•阿布力克木. N-烷氧基酰胺无溶剂氧化脱氢N—N自偶联反应研究[J]. 化学学报, 2024, 82(7): 731-735. |
| [3] | 张大伟, 赵海洋, 冯笑甜, 顾玉诚, 张新刚. 钯催化下杂芳基溴代物与偕二氟烯丙基硼试剂的交叉偶联反应[J]. 化学学报, 2024, 82(2): 105-109. |
| [4] | 刘建川, 李翠艳, 刘耀祖, 王钰杰, 方千荣. 高稳定二维联咔唑sp2碳共轭共价有机框架材料用于高效电催化氧还原★[J]. 化学学报, 2023, 81(8): 884-890. |
| [5] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [6] | 韩明亮, 徐丽华. 过渡金属催化的硫酯的交叉偶联反应研究进展[J]. 化学学报, 2023, 81(4): 381-392. |
| [7] | 王庆鑫, 崔勇, 李蕴琪, 卢善富, 相艳. Fe-N-C阴极催化层离聚物可控热解对膜电极性能与稳定性的影响研究★[J]. 化学学报, 2023, 81(10): 1350-1356. |
| [8] | 刘霞, 匡春香, 苏长会. 过渡金属催化的1,2,3-三氮唑导向的C—H键官能团化反应研究进展[J]. 化学学报, 2022, 80(8): 1135-1151. |
| [9] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| [10] | 曾誉, 吕品, 蔡跃进, 高福杰, 卓欧, 吴强, 杨立军, 王喜章, 胡征. 分级结构碳纳米笼高效催化苄胺氧化偶联制N-苄烯丁胺[J]. 化学学报, 2021, 79(4): 539-544. |
| [11] | 汪欣, 张贤睿, 黄宗煜, 樊新元, 陈鹏. 生物正交反应在我国的研究进展[J]. 化学学报, 2021, 79(4): 406-413. |
| [12] | 朱雪敏, 白小燕, 王海峰, 胡平, 汪必琴, 赵可清. 苯并䓛盘状液晶: 合成、柱状相和光物理性质[J]. 化学学报, 2021, 79(12): 1486-1493. |
| [13] | 袁宏宇, 徐敏敏, 姚建林. 电化学SPR协同催化对氯苯硫酚界面反应的SERS研究[J]. 化学学报, 2021, 79(12): 1481-1485. |
| [14] | 黄杰, 奚江波, 陈伟, 柏正武. 石墨烯衍生物作为无金属碳基催化剂在有机催化中的应用[J]. 化学学报, 2021, 79(11): 1360-1371. |
| [15] | 吴良, 魏瀚林, 申杰峰, 陈建中, 张万斌. 丰产金属催化烯基金属试剂的不对称烯基化反应研究进展[J]. 化学学报, 2021, 79(11): 1331-1344. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||