化学学报 ›› 2024, Vol. 82 ›› Issue (10): 1058-1068.DOI: 10.6023/A24080237 上一篇 下一篇
研究论文
投稿日期:2024-08-13
发布日期:2024-09-11
基金资助:Received:2024-08-13
Published:2024-09-11
Contact:
*E-mail: haiyanma@ecust.edu.cn; Tel.: 021-64253519
Supported by:文章分享
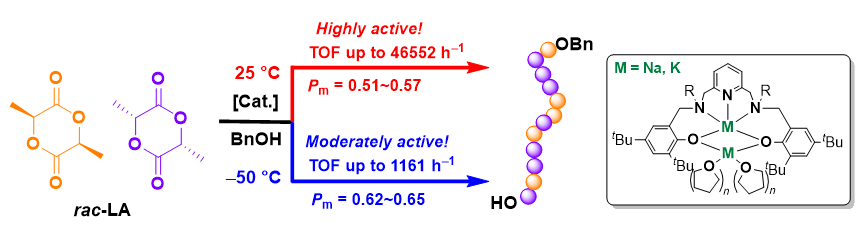
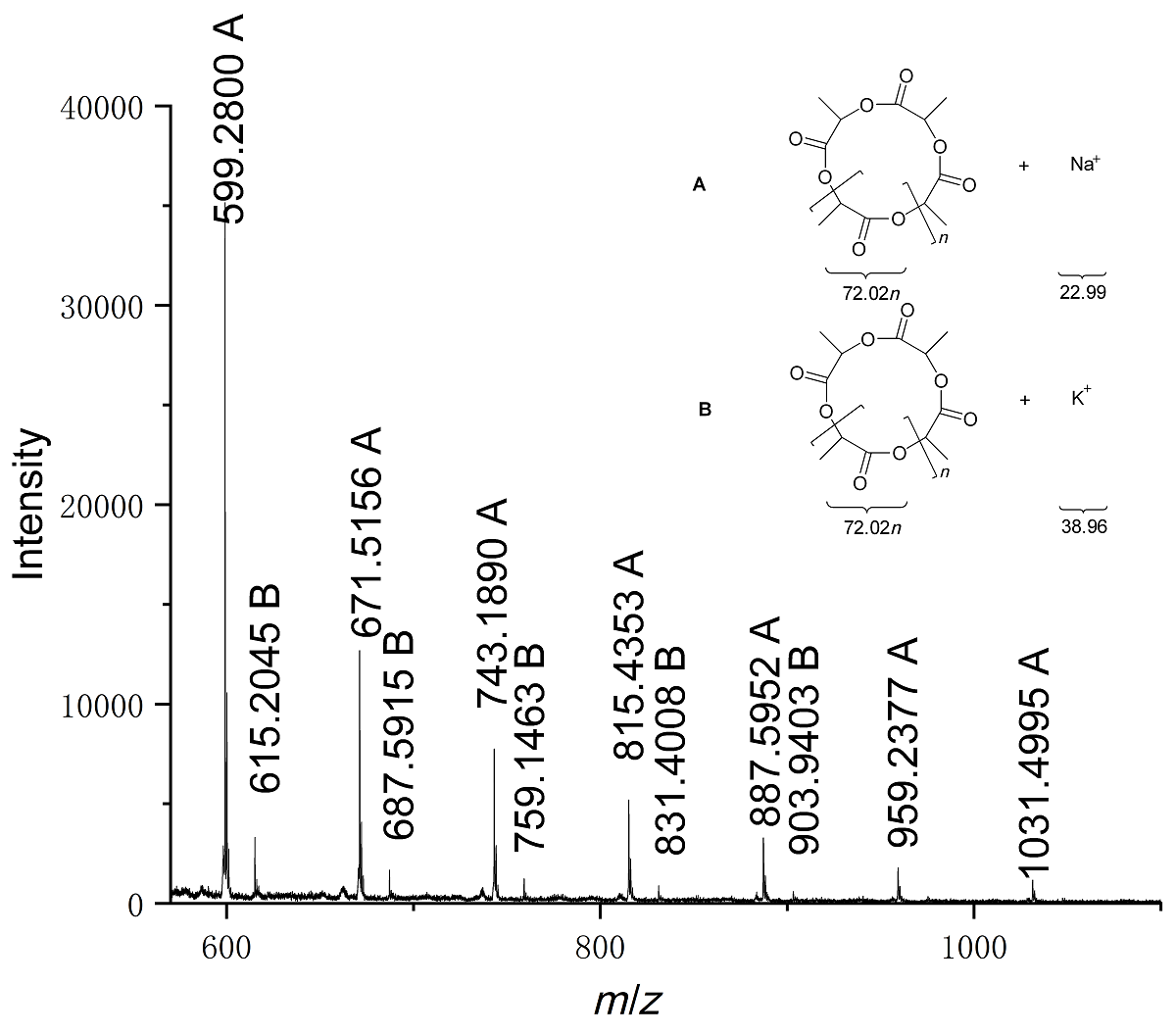
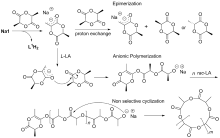
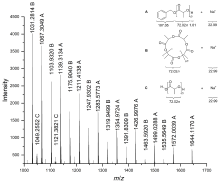
本工作基于2,6-二亚甲基吡啶桥联双(氨基酚)配体合成了四个新型双核钠、钾配合物, 通过1H NMR、13C NMR以及元素分析进行了表征. 典型钠配合物Na2通过X-ray单晶衍射分析确定具有双核结构, 其中一个钠中心与多齿配体所有杂原子成键形成五配位构型, 另一个钠中心除通过两个酚氧原子与前一钠中心桥联外, 还与两分子四氢呋喃配位形成四配位构型. 该类钠、钾配合物对外消旋丙交酯(racemic lactide, rac-LA)开环聚合具有很高的催化活性, 其中配体骨架氮上叔丁基取代的配合物Na3活性最高, 在室温、甲苯为溶剂, [rac-LA]0/[Cat.]0/[BnOH]0=500∶1∶4时, 转化频率(turnover frequency, TOF)值高达46552 h-1. 基于核磁跟踪以及基质辅助激光解吸飞行时间质谱(matrix-assisted laser desorption ionization time-of-flight mass spectrometry, MALDI-TOF MS)的研究结果, 认为不加醇条件下, 双核碱金属配合物易攫取丙交酯单体次甲基氢, 进而通过阴离子聚合机理催化丙交酯聚合; 而加入多倍量苄醇后, 阴离子过程受到部分抑制, 主要以配体辅助的单体活化机理进行聚合反应.
王镜焱, 马海燕. 2,6-二亚甲基吡啶桥联双(氨基酚氧基)钠、钾配合物的合成及催化外消旋丙交酯开环聚合研究[J]. 化学学报, 2024, 82(10): 1058-1068.
Jingyan Wang, Haiyan Ma. Syntheses of Sodium and Potassium Complexes Based on Pyridine-2,6-diyl-bis(methylene)-bridged Bis(aminophenolate) Ligands and Catalytic Ring-opening Polymerization of rac-Lactide[J]. Acta Chimica Sinica, 2024, 82(10): 1058-1068.
| Entry | Cat. | Feed Ratio | Temp./℃ | Time/min | Conv.b/% | TOFc/h-1 | Mn,calcdd/×104 | Mne/×104 | Mw/Mne | Pmf |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Na1 | 500∶1∶0 | 25 | 120 | 90 | 225 | 3.24 | 1.13 | 1.38 | 0.54 |
| 2 | Na1 | 500∶1∶1 | 25 | 2.5 | 90 | 10800 | 3.24 | 4.31 | 1.60 | 0.55 |
| 3 | Na1 | 500∶1∶2 | 25 | 1.1 | 85 | 22566 | 3.06 | 3.50 | 1.44 | 0.57 |
| 4 | Na1 | 500∶1∶4 | 25 | 0.67 | 91 | 40950 | 1.64 | 1.91 | 1.39 | 0.53 |
| 5g | Na1 | 500∶1∶2 | 25 | 0.70 | 95 | 40714 | 3.42 | 4.89 | 1.46 | ≈1 |
| 6 | Na1 | 500∶1∶2 | -50 | 28 | 61 | 663 | 2.20 | 2.21 | 1.21 | 0.65 |
| 7h | Na1 | 500∶1∶2 | -50 | 18 | 97 | 1622 | 3.51 | 3.89 | 1.62 | 0.62 |
| 8 | Na1 | 2000∶1∶2 | -50 | 47 | 37 | 955 | 5.33 | 4.81 | 1.48 | 0.63 |
| 9 | Na1 | 2000∶1∶5 | -50 | 47 | 45 | 1161 | 2.59 | 2.97 | 1.16 | 0.64 |
| 10 | Na2 | 500∶1∶2 | 25 | 1.5 | 91 | 18000 | 3.24 | 5.21 | 1.36 | 0.52 |
| 11 | Na2 | 500∶1∶4 | 25 | 0.83 | 95 | 34337 | 1.71 | 2.74 | 1.53 | 0.51 |
| 12 | Na2 | 500∶1∶2 | -50 | 136 | 90 | 198 | 3.24 | 6.66 | 1.45 | 0.63 |
| 13 | Na3 | 500∶1∶2 | 25 | 1.0 | 94 | 28200 | 3.39 | 4.56 | 1.43 | 0.56 |
| 14 | Na3 | 500∶1∶4 | 25 | 0.58 | 90 | 46552 | 1.62 | 1.96 | 1.41 | 0.54 |
| 15 | Na3 | 500∶1∶2 | -50 | 29 | 85 | 879 | 3.06 | 2.33 | 1.26 | 0.62 |
| 16 | K1 | 500∶1∶2 | 25 | 1.3 | 95 | 21923 | 3.42 | 6.31 | 1.49 | 0.54 |
| 17 | K1 | 500∶1∶4 | 25 | 0.90 | 90 | 40500 | 1.62 | 2.79 | 1.47 | 0.53 |
| 18 | K1 | 500∶1∶2 | -50 | 46 | 91 | 591 | 3.29 | 4.11 | 1.51 | 0.62 |
| Entry | Cat. | Feed Ratio | Temp./℃ | Time/min | Conv.b/% | TOFc/h-1 | Mn,calcdd/×104 | Mne/×104 | Mw/Mne | Pmf |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Na1 | 500∶1∶0 | 25 | 120 | 90 | 225 | 3.24 | 1.13 | 1.38 | 0.54 |
| 2 | Na1 | 500∶1∶1 | 25 | 2.5 | 90 | 10800 | 3.24 | 4.31 | 1.60 | 0.55 |
| 3 | Na1 | 500∶1∶2 | 25 | 1.1 | 85 | 22566 | 3.06 | 3.50 | 1.44 | 0.57 |
| 4 | Na1 | 500∶1∶4 | 25 | 0.67 | 91 | 40950 | 1.64 | 1.91 | 1.39 | 0.53 |
| 5g | Na1 | 500∶1∶2 | 25 | 0.70 | 95 | 40714 | 3.42 | 4.89 | 1.46 | ≈1 |
| 6 | Na1 | 500∶1∶2 | -50 | 28 | 61 | 663 | 2.20 | 2.21 | 1.21 | 0.65 |
| 7h | Na1 | 500∶1∶2 | -50 | 18 | 97 | 1622 | 3.51 | 3.89 | 1.62 | 0.62 |
| 8 | Na1 | 2000∶1∶2 | -50 | 47 | 37 | 955 | 5.33 | 4.81 | 1.48 | 0.63 |
| 9 | Na1 | 2000∶1∶5 | -50 | 47 | 45 | 1161 | 2.59 | 2.97 | 1.16 | 0.64 |
| 10 | Na2 | 500∶1∶2 | 25 | 1.5 | 91 | 18000 | 3.24 | 5.21 | 1.36 | 0.52 |
| 11 | Na2 | 500∶1∶4 | 25 | 0.83 | 95 | 34337 | 1.71 | 2.74 | 1.53 | 0.51 |
| 12 | Na2 | 500∶1∶2 | -50 | 136 | 90 | 198 | 3.24 | 6.66 | 1.45 | 0.63 |
| 13 | Na3 | 500∶1∶2 | 25 | 1.0 | 94 | 28200 | 3.39 | 4.56 | 1.43 | 0.56 |
| 14 | Na3 | 500∶1∶4 | 25 | 0.58 | 90 | 46552 | 1.62 | 1.96 | 1.41 | 0.54 |
| 15 | Na3 | 500∶1∶2 | -50 | 29 | 85 | 879 | 3.06 | 2.33 | 1.26 | 0.62 |
| 16 | K1 | 500∶1∶2 | 25 | 1.3 | 95 | 21923 | 3.42 | 6.31 | 1.49 | 0.54 |
| 17 | K1 | 500∶1∶4 | 25 | 0.90 | 90 | 40500 | 1.62 | 2.79 | 1.47 | 0.53 |
| 18 | K1 | 500∶1∶2 | -50 | 46 | 91 | 591 | 3.29 | 4.11 | 1.51 | 0.62 |

| [1] |
Garlotta, D. J. Polym. Environ. 2001, 9 63.
|
| [2] |
Fukushima, K.; Kimura, Y. Polym. Int. 2006, 55 626.
|
| [3] |
Thomas, C. M. Chem. Soc. Rev. 2010, 39 165.
|
| [4] |
Dijkstra, P. J.; Du, H.; Feijen, J. Polym. Chem. 2011, 2 520.
|
| [5] |
Thomas, C.; Lutz, J.-F. Angew. Chem. Int. Ed. 2011, 50 9244.
|
| [6] |
Brizzolara, D.; Cantow, H.-J.; Diederichs, K.; Keller, E.; Domb, A. J. Macromolecules 1996, 29 191.
|
| [7] |
Albertsson, A.-C.; Varma, I. K. Biomacromolecules 2003, 4 1466.
|
| [8] |
Pongpanit, T.; Saeteaw, T.; Chumsaeng, P.; Chasing, P.; Phomphrai, K. Inorg. Chem. 2021, 60 17114.
doi: 10.1021/acs.inorgchem.1c02382 pmid: 34605644 |
| [9] |
Vink, E. T. H.; Davies, S. Ind. Biotech. 2015, 11 167.
|
| [10] |
Auras, R.; Harte, B.; Selke, S. Macromol. Biosci. 2004, 4 835.
|
| [11] |
Hofmann, D.; Entrialgo-Castaño, M.; Kratz, K.; Lendlein, A. Adv. Mater. 2009, 21 3237.
|
| [12] |
Zhang, G.-D.; Yang, J.-Y.; Feng, X.-D.; Gu, Z.-W. Prog. Chem. 2000, 12 89 (in Chinese).
|
|
(张国栋, 杨纪元, 冯新德, 顾忠伟, 化学进展, 2000, 12 89.)
|
|
| [13] |
Le Borgne, A.; Vincens, V.; Jouglard, M.; Spassky, N. Makromol. Chem. 2011, 73 37.
|
| [14] |
Zhong, Z.; Dijkstra, P. J.; Feijen, J. Angew. Chem. Int. Ed. 2002, 41 4510.
|
| [15] |
Tang, Z.; Chen, X.; Pang, X.; Yang, X.; Zhang, X.; Jing, X. Biomacromolecules 2004, 5 965.
|
| [16] |
Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Chem.-Eur. J. 2007, 13 4433.
|
| [17] |
Chen, H.-L.; Dutta, S.; Huang, P.-Y.; Lin, C.-C. Organometallics 2012, 31 2016.
|
| [18] |
Maudoux, N.; Roisnel, T.; Dorcet, V.; Carpentier, J.-F. Chem.-Eur. J. 2014, 20 6131.
|
| [19] |
Myers, D.; White, A. J. P.; Forsyth, C. M.; Sarazin, Y. Angew. Chem. Int. Ed. 2017, 56 5277.
|
| [20] |
Yuntawattana, N.; Mcguire, T. M.; Durr, C. B.; Buchard, A.; Williams, C. K. Catal. Sci. Technol. 2020, 10 7226.
|
| [21] |
Pang, X.; Duan, R.; Li, X.; Hu, C.; Wang, X.; Chen, X. Macromolecules 2018, 51 906.
|
| [22] |
Wan, Y.; Bai, Y.; He, J.; Zhang, Y. Macromol. Rapid. Comm. 2020, 42 2000491.
|
| [23] |
Bhattacharjee, J.; Peters, M.; Bockfeld, D.; Tamm, M. Chem.-Eur. J. 2021, 27 5913.
doi: 10.1002/chem.202100482 pmid: 33555047 |
| [24] |
Cheng, M.; Attygalle, A. B.; Lobkovsky, E. B.; Coates, G. W. J. Am. Chem. Soc. 1999, 121 11583.
|
| [25] |
Malcolm, H.; Chisholm, J. C. G.; Zhen, H. Inorg. Chem. 2001, 40 5051.
pmid: 11531458 |
| [26] |
Williams, C. K.; Brooks, N. R.; Hillmyer, M. A.; Tolman, W. B. Chem. Commun. 2002, 18 2132.
|
| [27] |
Malcolm, H.; Chisholm, J. C. G.; Khamphee, P. Inorg. Chem. 2004, 43 6717.
pmid: 15476371 |
| [28] |
Wu, J.-C.; Huang, B.-H.; Hsueh, M.-L.; Lai, S.-L.; Lin, C.-C. Polymer 2005, 46 9784.
|
| [29] |
Abbina, S.; Du, G. ACS Macro Lett. 2014, 3 689.
|
| [30] |
Wang, H.; Ma, H. Chem. Commun. 2013, 49 8686.
|
| [31] |
Kan, C.; Hu, J.; Huang, Y.; Wang, H.; Ma, H. Macromolecules 2017, 50 7911.
|
| [32] |
Hu, J.; Kan, C.; Wang, H.; Ma, H. Macromolecules 2018, 51 5304.
|
| [33] |
Gong, Y.; Ma, H. Chem. Commun. 2019, 55 10112.
|
| [34] |
Ma, H.; Jiang, X. CN111362885. 2020, 173 487229]
|
|
(马海燕, 蒋旭敏, CN111362885. 2020, 173 487229]).
|
|
| [35] |
Ma, H.; Shao, M. CN113264901. 2021, 176 93892]
|
|
(马海燕, 邵猛, CN113264901. 2021, 176 93892]).
|
|
| [36] |
Ma, H.; Gong, S. CN114349781. 2022, 178 368641]
|
|
(马海燕, 龚闪闪, CN114349781. 2022, 178 368641]).
|
|
| [37] |
Bhattacharjee, J.; Harinath, A.; Nayek, H. P.; Sarkar, A.; Panda, T. K. Chem.-Eur. J. 2017, 23 9319.
doi: 10.1002/chem.201700672 pmid: 28493433 |
| [38] |
Rosen, T.; Rajpurohit, J.; Lipstman, S.; Venditto, V.; Kol, M. Chem.-Eur. J. 2020, 26 17183.
|
| [39] |
Chellali, J. E.; Alverson, A. K.; Robinson, J. R. ACS Catal. 2022, 12 5585.
|
| [40] |
Liu, J.-Y.; Liu, S.; Suo, H.-Y.; Qin, Y.-S. Acta Polym. Sin. 2024, 55 770 (in Chinese).
|
|
(刘娇玉, 刘爽, 索泓一, 秦玉升, 高分子学报, 2024, 55 770.)
|
|
| [41] |
Ma, H.; Spaniol, T. P.; Okuda, J. Angew. Chem. Int. Ed. 2006, 118 7982.
|
| [42] |
Buffet, J. C.; Kapelski, A.; Okuda, J. Macromolecules 2010, 43 10201.
|
| [43] |
Liu, X.; Shang, X.; Tang, T.; Hu, N.; Pei, F.; Cui, D.; Chen, X.; Jing, X. Organometallics 2007, 26 2747.
|
| [44] |
Luo, Y.; Li, W.; Lin, D.; Yao, Y.; Zhang, Y.; Shen, Q. Organometallics 2010, 29 3507.
|
| [45] |
Yang, S.; Du, Z.; Zhang, Y.; Shen, Q. Chem. Commun. 2012, 48 9780.
|
| [46] |
Bouyahyi, M.; Ajellal, N.; Kirillov, E.; Thomas, C. M.; Carpentier, J.-F. Chem.-Eur. J. 2011, 17 1872.
doi: 10.1002/chem.201002779 pmid: 21274938 |
| [47] |
Nie, K.; Fang, L.; Yao, Y.; Zhang, Y.; Shen, Q.; Wang, Y. Inorg. Chem. 2012, 51 11133.
|
| [48] |
Xu, T.-Q.; Yang, G.-W.; Liu, C.; Lu, X. Macromolecules 2017, 50 515.
|
| [49] |
Duan, Y.-L.; Guo, Q.; Liu, G.-Y.; Yi, Z.-Z.; Feng, S.-P.; Huang, Y. Polym. Chem. 2022, 13 4249.
|
| [50] |
Ko, B.-T.; Lin, C.-C. J. Am. Chem. Soc. 2001, 123 7973.
pmid: 11506552 |
| [51] |
Hsueh, M.-L.; Huang, B.-H.; Wu, J.; Lin, C.-C. Macromolecules 2005, 38 9482.
|
| [52] |
Chen, H.-Y.; Zhang, J.; Lin, C.-C.; Reibenspies, J.-H.; Miller, S.-A. Green Chem. 2007, 9 1038.
|
| [53] |
Huang, Y.; Tsai, Y.-H.; Hung, W.-C.; Lin, C.-S.; Wang, W.; Huang, J.-H.; Dutta, S.; Lin, C.-C. Inorg. Chem. 2010, 49 9416.
doi: 10.1021/ic1011154 pmid: 20843075 |
| [54] |
Huang, Y.; Wang, W.; Lin, C.-C.; Blake, M. P.; Clark, L.; Schwarz, A. D.; Mountford, P. Dalton Trans. 2013, 42 9313.
doi: 10.1039/c3dt50135c pmid: 23435514 |
| [55] |
García-valle, F. M.; Estivill, R.; Gallegos, C.; Mosquera, M. E. G.; Tabernero, V.; Cano, J. Organometallics 2015, 34 477.
|
| [56] |
Dean, R. K.; Reckling, A. M.; Chen, H.; Dawe, L. N.; Schneider, C. M.; Kozak, C. M. Dalton Trans. 2013, 42 3504.
|
| [57] |
Alhashmialameer, D.; Ikpo, N.; Collins, J.; Dawe, L. N.; Hattenhauer, K.; Kerton, F. M. Dalton Trans. 2015, 44 20216.
doi: 10.1039/c5dt03119b pmid: 26538475 |
| [58] |
Yao, C.; Yang, Y.; Xu, S.; Ma, H. Dalton Trans. 2017, 46 6087.
|
| [59] |
Ma, H.; Hu, J.; Huang, Y. CN108047256. 2018, 169 46195]
|
|
(马海燕, 胡建文, 黄洋, CN108047256. 2018, 169 46195]).
|
|
| [60] |
Ma, H.; Li, H.; Huang, Y. CN116854712. 2023, 185 211761]
|
|
(马海燕, 李和华, 黄洋, CN116854712. 2023, 185 211761]).
|
|
| [61] |
Ma, H.; Huang, Y.; Li, H. CN116874414. 2023, 185 30640]
|
|
(马海燕, 黄洋, 李和华, CN116874414. 2023, 185 30640]).
|
|
| [62] |
Zhang, J.; Xiong, J.; Sun, Y.; Dai, Z.; Pan, X.; Wu, J. Macromolecules 2014, 47 7789.
|
| [63] |
Xiong, J.; Zhang, J.; Sun, Y.; Xiong, J.; Pan, X.; Wu, J. Inorg. Chem. 2015, 54 1737.
doi: 10.1021/ic502685f pmid: 25597469 |
| [64] |
Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. ACS Macro Lett. 2015, 4 556.
|
| [65] |
Sun, Y.; Xiong, J.; Dai, Z.; Pan, X.; Tang, N.; Wu, J. Inorg. Chem. 2015, 55 136.
|
| [66] |
Dai, Z.; Sun, Y.; Xiong, J.; Pan, X.; Tang, N.; Wu, J. Catal. Sci. Technol. 2016, 6 515.
|
| [67] |
Chen, C.; Cui, Y.; Mao, X.; Pan, X.; Wu, J. Macromolecules 2016, 50 83.
|
| [68] |
Cui, Y.; Chen, C.; Sun, Y.; Wu, J.; Pan, X. Inorg. Chem. Front. 2017, 4 261.
|
| [69] |
Chen, C.; Jiang, J.; Mao, X.; Cong, Y.; Cui, Y.; Pan, X.; Wu, J. Inorg. Chem. 2018, 57 3158.
|
| [70] |
Wu, B.-B.; Tian, L.-L.; Wang, Z.-X. RSC Adv. 2017, 7 24055.
|
| [71] |
Fernández-Millán, M.; Ortega, P.; Cuenca, T.; Cano, J.; Mosquera, M. E. G. Organometallics 2020, 39 2278.
|
| [72] |
Cui, Y.; Jiang, J.; Mao, X.; Wu, J. Inorg. Chem. 2019, 58 218.
|
| [73] |
Li, X.; Jia, Z.; Pan, X.; Wu, J. Chem. Asian J. 2019, 14 662.
|
| [74] |
Harinath, A.; Bhattacharjee, J.; Sarkar, A.; Panda, T. K. New J. Chem. 2019, 43 8882.
|
| [75] |
Ren, F.; Li, X.; Xian, J.; Han, X.; Cao, L.; Pan, X.; Wu, J. J. Polym. Sci. 2022, 60 2847.
|
| [76] |
Stopper, A.; Press, K.; Okuda, J.; Goldberg, I.; Kol, M. Inorg. Chem. 2014, 53 9140.
|
| [77] |
Jeong, Y.; Shin, M.; Seo, M.; Kim, H. Organometallics 2022, 41 328.
|
| [78] |
Stewart, J. A.; Mckeown, P.; Driscoll, O. J.; Mahon, M. F.; Ward, B. D.; Jones, M. D. Macromolecules 2019, 52 5977.
doi: 10.1021/acs.macromol.9b01205 |
| [79] |
Marin, P.; Tschan, M. J. L.; Isnard, F.; Robert, C.; Haquette, P.; Trivelli, X.; Chamoreau, L. M.; Guérineau, V.; Rosal, I. D.; Maron, L.; Venditto, V.; Thomas, C. Angew. Chem. Int. Ed. 2019, 58 12585.
doi: 10.1002/anie.201903224 pmid: 30908800 |
| [80] |
Lee, J.; Nayab, S.; Kumar, A.; Kim, D.; Jung, H.; Lee, S.-H.; Cho, D.; Lee, H. Bull. Korean Chem. Soc. 2024, 45 317.
|
| [81] |
Baker, C. A.; Romain, C.; Long, N. J. Chem. Commun. 2021, 57 12524.
|
| [82] |
Liu, S.; Li, H.; Zhao, N.; Li, Z. ACS Macro Lett. 2018, 7 624.
|
| [83] |
Liu, Y.; Zhang, J.; Kou, X.; Liu, S.; Li, Z. ACS Macro Lett. 2022, 11 1183.
|
| [84] |
Zaky, M. S.; Wirotius, A.-L.; Coulembier, O.; Guichard, G.; Taton, D. ACS Macro Lett. 2022, 11 1148.
|
| [85] |
Zaky, M. S.; Guichard, G.; Taton, D. Macromolecules 2023, 56 3607.
|
| [86] |
Kou, X.-H.; Shen, Y.; Li, Z.-B. Acta Polym. Sin. 2020, 51 1121 (in Chinese).
|
|
(寇新慧, 沈勇, 李志波, 高分子学报, 2020, 51 1121.)
|
|
| [87] |
Li, H.-Q.; Wang, J.-Y.; Wu, L.; Liu, W.; Cheng, R.-H.; Liu, B.-P. Acta Polym. Sin. 2019, 50 1290 (in Chinese).
|
|
(李海强, 汪婧怡, 武莉, 刘威, 程瑞华, 刘柏平, 高分子学报, 2019, 50 1290.)
|
|
| [88] |
Ghosh, S.; Schulte, Y.; Wölper, C.; Tjaberings, A.; Gröschel, A. H.; Haberhauer, G.; Schulz, S. Organometallics 2022, 41 2698.
|
| [89] |
Kremer, A. B.; Mehrkhodavandi, P. Coord. Chem. Rev. 2019, 380 35.
|
| [90] |
Williams, C. K.; Breyfogle, L. E.; Choi, S. K.; Nam, W.; Young Jr, V. G.; Hillmyer, M. A.; Tolman, W. B. J. Am. Chem. Soc. 2003, 125 11350.
|
| [91] |
Gruszka, W.; Walker, L. C.; Shaver, M. P.; Garden, J. A. Macromolecules 2020, 53 4294.
|
| [92] |
Jadrich, C. N.; Pane, V. E.; Lin, B.-H.; Jones, G. O.; Hedrick, J. L.; Park, N. H.; Waymouth, R. M. J. Am. Chem. Soc. 2022, 144 8439.
doi: 10.1021/jacs.2c01436 pmid: 35504294 |
| [93] |
Ovitt, T. M.; Coates, G. W. J. Am. Chem. Soc. 2002, 124 1316.
pmid: 11841301 |
| [94] |
Appiah, W. O.; DeGreeff, A. D.; Razidlo, G. L.; Spessard, S. J.; Pink, M.; Young, V. G.; Hofmeister, G. E. Inorg. Chem. 2002, 41 3656.
|
| [95] |
Chow, H. S.; Constable, E. C.; Housecroft, C. E.; Neuburger, M.; Schaffner, S. Dalton Trans. 2006, 23 2881.
|
| [96] |
Jalee, K.; Bongki, S.; Hyunjeong, K.; Junhyung, L.; Joongoo, K.; Sachiko, Y.; Takashi, O.; Hideki, M.; Tomohiro, O.; Jaeheung, C. Inorg. Chem. 2015, 54 6176.
doi: 10.1021/acs.inorgchem.5b00294 pmid: 26068376 |
| [97] |
Che, C.-M.; Li, Z.-Y.; Wong, K.-Y.; Poon, C.-K.; Mak, T. C. W.; Peng, S.-M. Polyhedron 1994, 13 771.
|
| [1] | 杨贯文, 伍广朋. 模块化双功能有机硼氮和硼磷催化体系的设计及其催化转化★[J]. 化学学报, 2023, 81(11): 1551-1565. |
| [2] | 江金辉, 朱云卿, 杜建忠. 开环聚合诱导自组装的挑战与展望[J]. 化学学报, 2020, 78(8): 719-724. |
| [3] | 李荣烨, Khiman Mehul, 盛力, 孙静. 两亲性聚氨基酸三嵌段共聚物构筑pH/溶剂可控多级纳米结构[J]. 化学学报, 2020, 78(11): 1235-1239. |
| [4] | 布美热木·克力木, 马海燕. 非对称β-二亚氨基镁络合物催化丙交酯、己内酯开环聚合/共聚研究[J]. 化学学报, 2018, 76(2): 121-132. |
| [5] | 张弛, 李杰, 罗运军, 葛震. 微波合成3,3'-双叠氮甲基环氧丁烷-3-叠氮甲基-3'-甲基环氧丁烷无规共聚物[J]. 化学学报, 2012, 0(04): 492-498. |
| [6] | 胡承波, 傅亚, 向鸿照, 孙娇霞, 阮长顺, 李香, 向燕, 彭琴, 王远亮. 双(烷氧-亚胺芳氧)基钛(IV)配合物催化D,L-丙交酯开环聚合、动力学及机理[J]. 化学学报, 2011, 69(21): 2574-2582. |
| [7] | 李启蒸, 张国艺, 黄晋, 赵巧玲, 魏柳荷, 何占航, 马志. 结构可控的聚亚甲基/聚乳酸嵌段共聚物的合成及其性能研究[J]. 化学学报, 2011, 69(04): 497-502. |
| [8] | 徐旭,伍国琳,张洁,王亦农,范云鸽,马建标. 具有乙二醇侧链的聚谷氨酸酯的合成、表征及其两亲性[J]. 化学学报, 2008, 66(9): 1102-1106. |
| [9] | 朱荣秀,张冬菊,王若曦,刘成卜. 双官能团硫脲催化丙交酯开环聚合反应的理论研究[J]. 化学学报, 2008, 66(8): 885-889. |
| [10] | 华佳捷,杨建,胡艳飞,韦嘉,李速明. 低毒锌类催化剂制备聚乳酸的研究[J]. 化学学报, 2008, 66(24): 2730-2734. |
| [11] | 杨纪元,余坚,李梅,潘怀忠,顾忠伟,曹维孝,冯新德. 新型两亲性生物降解聚酯单体的合成及其开环聚合[J]. 化学学报, 2001, 59(10): 1809-1812. |
| [12] | 沈之荃,孙俊全,吴良江,吴兰亭. 稀土配位催化合成聚乳酸[J]. 化学学报, 1990, 48(7): 686-689. |
| [13] | 刘德海,李露,闵利,刘道玉. 有机钼, 钨化合物研究IV:新的环烯开环聚合催化体系及聚合物性质的初步研究[J]. 化学学报, 1986, 44(12): 1224-1228. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
