化学学报 ›› 2025, Vol. 83 ›› Issue (6): 596-601.DOI: 10.6023/A25030064 上一篇 下一篇
研究论文
李文静*( ), 杨黎燕, 关丽, 张雪娇, 尤静, 沈思语, 赵钰琦, 段琛
), 杨黎燕, 关丽, 张雪娇, 尤静, 沈思语, 赵钰琦, 段琛
投稿日期:2025-03-04
发布日期:2025-05-08
基金资助:
Wenjing Li*( ), Liyan Yang, Li Guan, Xuejiao Zhang, Jing You, Siyu Shen, Yuqi Zhao, Chen Duan
), Liyan Yang, Li Guan, Xuejiao Zhang, Jing You, Siyu Shen, Yuqi Zhao, Chen Duan
Received:2025-03-04
Published:2025-05-08
Contact:
*E-mail: liwenjing@xiyi.edu.cn
Supported by:文章分享
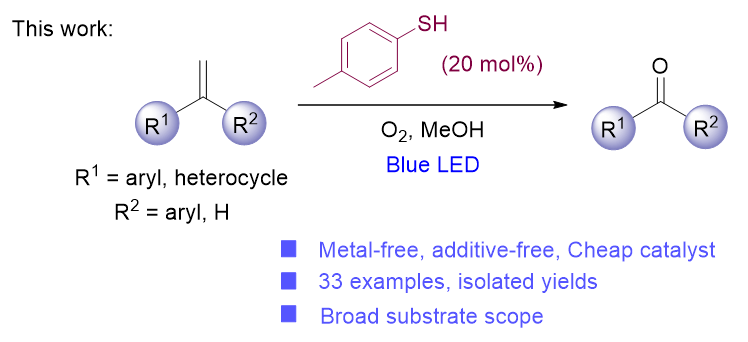
芳香酮类化合物在药物合成、化学生物技术、染料设计和功能材料制备等领域具有广阔的应用前景. 在各种合成方法中, 通过烯烃直接氧化裂解获取芳香酮是一种经典而简便的方法. 本工作开发了一种温和、高效的烯烃氧化裂解方法, 在可见光照射下, 以烯烃为原料、4-甲苯硫酚为有机小分子光催化剂、氧气为氧化剂、甲醇为溶剂, 以较高产率获得了一系列芳基酮衍生物. 该体系具有产率高、操作简单、反应条件温和、广泛的底物范围和良好的官能团耐受性等优点. 此外, 克级实验证明了该方法在酮类放大合成中的应用前景, 且在最优条件下高产率合成了药物分子酮洛芬甲基酯和非诺贝特. 最后, 通过控制实验研究了单线态氧对反应的影响, 并提出了合理的催化循环机理.
李文静, 杨黎燕, 关丽, 张雪娇, 尤静, 沈思语, 赵钰琦, 段琛. 可见光/硫酚催化烯烃C=C双键的氧化裂解反应[J]. 化学学报, 2025, 83(6): 596-601.
Wenjing Li, Liyan Yang, Li Guan, Xuejiao Zhang, Jing You, Siyu Shen, Yuqi Zhao, Chen Duan. Thiophenol-Catalyzed Visible-Light-Mediated Oxidative Cleavage of C=C Bond of Alkene[J]. Acta Chimica Sinica, 2025, 83(6): 596-601.
| Entry | Photocatalyst | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | [Mes-Acr+-Me$ClO_{4}^{-}$] | CH3CN | 20 |
| 2 | Ethidium bromide | CH3CN | 26 |
| 3 | 2,4,6-Triphenylpyrylium Tetrafluoroborate | CH3CN | 18 |
| 4 | Eosin B | CH3CN | 21 |
| 5 | Eosin YS | CH3CN | 18 |
| 6 | Rhodamine B | CH3CN | 19 |
| 7 | p-toluenethiol | CH3CN | 46 |
| 8 | p-toluenethiol | H2O | 32 |
| 9 | p-toluenethiol | DMAc | 39 |
| 10 | p-toluenethiol | DMSO | 45 |
| 11 | p-toluenethiol | DCE | 53 |
| 12 | p-toluenethiol | THF | 43 |
| 13 | p-toluenethiol | 1,4-Dioxane | 51 |
| 14 | p-toluenethiol | IPA | 60 |
| 15 | p-toluenethiol | EtOH | 54 |
| 16 | p-toluenethiol | MeOH | 63 |
| 17c | p-toluenethiol | MeOH | 85 |
| Entry | Photocatalyst | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | [Mes-Acr+-Me$ClO_{4}^{-}$] | CH3CN | 20 |
| 2 | Ethidium bromide | CH3CN | 26 |
| 3 | 2,4,6-Triphenylpyrylium Tetrafluoroborate | CH3CN | 18 |
| 4 | Eosin B | CH3CN | 21 |
| 5 | Eosin YS | CH3CN | 18 |
| 6 | Rhodamine B | CH3CN | 19 |
| 7 | p-toluenethiol | CH3CN | 46 |
| 8 | p-toluenethiol | H2O | 32 |
| 9 | p-toluenethiol | DMAc | 39 |
| 10 | p-toluenethiol | DMSO | 45 |
| 11 | p-toluenethiol | DCE | 53 |
| 12 | p-toluenethiol | THF | 43 |
| 13 | p-toluenethiol | 1,4-Dioxane | 51 |
| 14 | p-toluenethiol | IPA | 60 |
| 15 | p-toluenethiol | EtOH | 54 |
| 16 | p-toluenethiol | MeOH | 63 |
| 17c | p-toluenethiol | MeOH | 85 |
| [1] |
|
| [2] |
(a)
doi: 10.1002/anie.201301124 pmid: 18505290 |
|
(b)
doi: 10.1021/ol300617r pmid: 18505290 |
|
|
(c)
pmid: 18505290 |
|
|
(d)
doi: 10.1021/jo800323x pmid: 18505290 |
|
|
(e)
pmid: 18505290 |
|
| [3] |
|
| [4] |
(a)
pmid: 27783071 |
|
(b)
pmid: 27783071 |
|
|
(c)
pmid: 27783071 |
|
|
(d)
pmid: 27783071 |
|
|
(e)
pmid: 27783071 |
|
| [5] |
|
| [6] |
|
| [7] |
(a)
|
|
(b)
|
|
| [8] |
(a)
pmid: 26027938 |
|
(b)
pmid: 26027938 |
|
|
(c)
doi: 10.1021/jacs.5b03956 pmid: 26027938 |
|
|
(d)
pmid: 26027938 |
|
| [9] |
(a)
pmid: 31244154 |
|
(b)
pmid: 31244154 |
|
|
(c)
doi: 10.1021/acs.joc.9b00487 pmid: 31244154 |
|
| [10] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
doi: 10.1039/c8qo01412d |
|
| [11] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
|
(h)
|
|
| [12] |
(a)
pmid: 35930615 |
|
(b)
doi: 10.1021/jacs.2c05648 pmid: 35930615 |
|
|
(c)
pmid: 35930615 |
|
| [13] |
|
| [14] |
|
| [15] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
|
|
(g)
|
|
| [16] |
(a)
pmid: 11421768 |
|
(b)
pmid: 11421768 |
|
| [17] |
|
| [18] |
|
| [19] |
(a)
|
|
(b)
|
|
| [20] |
pmid: 2226216 |
| [21] |
(a)
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
(f)
|
| [1] | 胡博, 肖霞, 王鹏, 束小龙, 卞梦琪, 王健捷, 赵震. 二氧化碳加氢制低碳烯烃Fe基催化剂研究进展[J]. 化学学报, 2025, 83(5): 535-550. |
| [2] | 杨青, 刘肖宇, 王晨, 徐敏敏, 姚建林. 金纳米粒子-金单晶微米片自由碰撞行为中“热点”限域空间内的表面增强拉曼光谱研究[J]. 化学学报, 2024, 82(3): 281-286. |
| [3] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [4] | 万义, 何江华, 张越涛. Lewis酸碱对催化极性烯烃单体精准聚合的研究进展★[J]. 化学学报, 2023, 81(9): 1215-1230. |
| [5] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [6] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [7] | 袁芳艳, 李超, 罗美明, 曾小明. 铬催化酮羰基的脱氧偶联反应合成四取代烯烃★[J]. 化学学报, 2023, 81(5): 456-460. |
| [8] | 汪阳, 阎敬灵. 不同配体的稀土金属配合物在烯烃聚合领域的研究进展[J]. 化学学报, 2023, 81(3): 275-288. |
| [9] | 陈治平, 孟永乐, 芦静, 周文武, 杨志远, 周安宁. Fe@Si/S-34催化剂的制备及其合成气制烯烃性能[J]. 化学学报, 2023, 81(1): 14-19. |
| [10] | 王玉银, 胡小强, 穆红亮, 夏艳, 迟悦, 简忠保. 空间位阻与氟效应协同增强镍系乙烯聚合[J]. 化学学报, 2022, 80(6): 741-747. |
| [11] | 杨民, 叶柏柏, 陈健强, 吴劼. 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022, 80(1): 11-15. |
| [12] | 马智烨, 叶丽, 吴雨桓, 赵彤. B,N-SnO2/TiO2光催化剂的制备及其光催化性能研究[J]. 化学学报, 2021, 79(9): 1173-1179. |
| [13] | 赵庆如, 蒋茹, 游书力. 铱催化串联不对称烯丙基取代/双键异构化构建轴手性化合物[J]. 化学学报, 2021, 79(9): 1107-1112. |
| [14] | 麻旺坪, 贺彦彦, 刘洪来. 烯烃连接的三嗪共轭多孔聚合物的合成及其可见光催化产氢性能[J]. 化学学报, 2021, 79(7): 914-919. |
| [15] | 袁宏宇, 徐敏敏, 姚建林. 电化学SPR协同催化对氯苯硫酚界面反应的SERS研究[J]. 化学学报, 2021, 79(12): 1481-1485. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||