化学学报 ›› 2026, Vol. 84 ›› Issue (1): 86-92.DOI: 10.6023/A25070255 上一篇 下一篇
研究论文
杨凯a, 吴龙辉a, 付鑫磊a, 单申a, 吴汉清b,*( ), 黄九忠a,*(
), 黄九忠a,*( )
)
投稿日期:2025-07-16
发布日期:2025-09-10
基金资助:
Kai Yanga, Longhui Wua, Xinlei Fua, Shen Shana, Hanqing Wub,*( ), Jiuzhong Huanga,*(
), Jiuzhong Huanga,*( )
)
Received:2025-07-16
Published:2025-09-10
Contact:
* E-mail: wuhanqing12@foxmail.com;huangjz@gmu.edu.cn
Supported by:文章分享
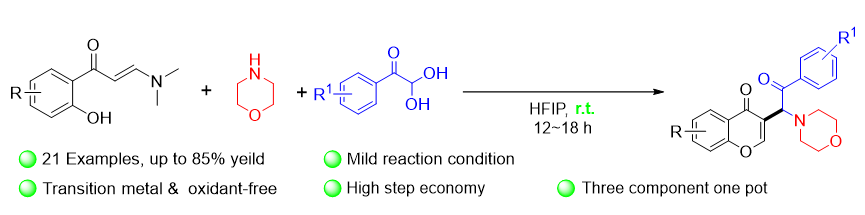
色酮衍生物广泛存在于天然产物和生物活性的分子中, 也是有机合成的重要砌块. 尽管基于色酮骨架分子合成研究已取得一定进展, 但利用砌块化学策略探索色酮类衍生物的新合成方法, 仍具有重要意义. 基于此, 以六氟异丙醇(HFIP)为溶剂, 通过邻羟基芳基烯胺酮、吗啉和芳基乙二醛的三组分串联环化反应, 可实现C3官能团化色酮衍生物的合成. 该反应体系以HFIP为溶剂和促进剂, 在无过渡金属催化条件下即可实现室温反应, 具有原料易得、操作简便、良好官能团兼容性等优势.
杨凯, 吴龙辉, 付鑫磊, 单申, 吴汉清, 黄九忠. 邻羟基芳基烯胺酮构建色酮衍生物的三组分串联环化反应研究[J]. 化学学报, 2026, 84(1): 86-92.
Kai Yang, Longhui Wu, Xinlei Fu, Shen Shan, Hanqing Wu, Jiuzhong Huang. Three-Component Tandem Cyclization for the Synthesis of Chromone Derivatives from ortho-Hydroxyaryl Enaminones[J]. Acta Chimica Sinica, 2026, 84(1): 86-92.
| Entry | Catalyst/Promoter | Solvent | 4a yieldb/% |
|---|---|---|---|
| 1 | — | DCM | trace |
| 2 | — | Toluene | 45 |
| 3 | — | IPA | 40 |
| 4 | — | 1,4-Dioxane | 37 |
| 5 | — | HFIP | 55 |
| 6 | — | DMF | 43 |
| 7 | — | Toluene/HFIP (1∶1) | 51 |
| 8c | — | HFIP | 61 |
| 9d | — | HFIP | 70 |
| 10d | TFA (20 mmol%) | HFIP | 66 |
| 11d | Y(OTf)3 (20 mmol%) | HFIP | 68 |
| 12d | Sc(OTf)3 (20 mmol%) | HFIP | 62 |
| 13d | TfOH (20 mmol%) | HFIP | 65 |
| 14d,e | — | HFIP | 75 |
| 15d,f | — | HFIP | 85 |
| 16d,g | — | HFIP | 84 |
| Entry | Catalyst/Promoter | Solvent | 4a yieldb/% |
|---|---|---|---|
| 1 | — | DCM | trace |
| 2 | — | Toluene | 45 |
| 3 | — | IPA | 40 |
| 4 | — | 1,4-Dioxane | 37 |
| 5 | — | HFIP | 55 |
| 6 | — | DMF | 43 |
| 7 | — | Toluene/HFIP (1∶1) | 51 |
| 8c | — | HFIP | 61 |
| 9d | — | HFIP | 70 |
| 10d | TFA (20 mmol%) | HFIP | 66 |
| 11d | Y(OTf)3 (20 mmol%) | HFIP | 68 |
| 12d | Sc(OTf)3 (20 mmol%) | HFIP | 62 |
| 13d | TfOH (20 mmol%) | HFIP | 65 |
| 14d,e | — | HFIP | 75 |
| 15d,f | — | HFIP | 85 |
| 16d,g | — | HFIP | 84 |
| [8] |
(c)
doi: 10.1021/acs.orglett.4c00528 |
| [9] |
doi: 10.1016/j.cclet.2024.110784 |
| [10] |
(a)
pmid: 35848353 |
|
(b)
doi: 10.1021/acs.chemrev.1c00749 pmid: 35848353 |
|
| [11] |
doi: 10.1016/j.tet.2024.133948 |
| [12] |
doi: 10.1039/D3OB01582C |
| [13] |
doi: 10.1002/chem.v25.72 |
| [14] |
(a)
doi: 10.1021/acs.joc.3c00093 |
|
(b)
doi: 10.1021/acssuschemeng.3c03467 |
|
|
(c)
doi: 10.1002/ejoc.v2022.32 |
|
|
(d)
doi: 10.1039/D0QO00065E |
|
|
(e)
|
|
| [15] |
(a)
doi: 10.1021/acs.joc.0c01207 |
|
(b)
doi: 10.1021/acs.joc.2c02812 |
|
| [1] |
(a)
doi: 10.1002/tcr.v23.11 |
|
(b)
doi: 10.6023/A23040192 |
|
|
(杨爽, 王宁宜, 杭青青, 张宇辰, 石枫, 化学学报, 2023, 81, 793.)
|
|
| [2] |
(a)
doi: 10.1021/acs.jmedchem.6b01720 |
|
(b)
doi: 10.1039/D4QO00677A |
|
|
(c)
doi: 10.1002/adsc.v367.6 |
|
|
(d)
doi: 10.1021/acsomega.4c00771 |
|
|
(e)
doi: 10.6023/cjoc202411009 |
|
|
(许秋玲, 孙瑞芬, 王均亮, 何晓山, 有机化学, 2025, 45, 2416.)
|
|
| [3] |
(a)
doi: 10.1007/s00044-025-03380-x |
|
(b)
doi: 10.1016/j.bioorg.2025.108338 |
|
|
(c)
|
|
| [4] |
(a)
doi: 10.1002/adsc.v367.10 |
|
(b)
doi: 10.1021/acs.orglett.0c01671 |
|
| [5] |
(a)
doi: 10.1002/cmdc.v15.5 |
|
(b)
doi: 10.1021/acschemneuro.0c00729 |
|
| [6] |
(a)
|
|
(b)
doi: 10.6023/cjoc202307014 |
|
|
(黄志友, 杨平, 何波, 欧文霞, 袁思雨, 有机化学, 2024, 44, 309.)
|
|
| [7] |
doi: 10.1002/med.v40.2 |
| [8] |
(a)
doi: 10.6023/cjoc201907018 |
|
(徐海伟, 李媛媛, 董航歧, 孟夏, 赵玲洁, 吕春涛, 王振亚, 金成允, 有机化学, 2020, 40, 69.)
|
|
|
(b)
doi: 10.6023/cjoc201912033 |
|
|
(朱仲珍, 乔雨, 张子豪, 顾明震, 王晋, 高志宇, 郭文昊, 刘明明, 李荣, 有机化学, 2020, 40, 1983.)
|
| [1] | 王超峰, 郑国栋, 王悦, 宋慧佳, 陈小艺, 高瑞霞. 金属卟啉-Sn网络可控非共价功能化碳纳米管的制备及蛋白吸附应用[J]. 化学学报, 2022, 80(2): 126-132. |
| [2] | 占林俊, 胡玮, 王梅, 黄斌, 龙亚秋. 亚胺氯化物介导一锅法合成3-吸电子基团取代吲哚衍生物[J]. 化学学报, 2021, 79(7): 903-907. |
| [3] | 李金华, 卓庆德, 卓凯玥, 陈大发, 夏海平. 铱杂碳龙配合物的合成及反应性[J]. 化学学报, 2021, 79(1): 71-80. |
| [4] | 唐敏, 吴永, 刘源, 蔡茂强, 夏飞, 刘顺英, 胡文浩. “一锅法”不对称多组分串联反应合成手性氢化环氧异色烯衍生物:一种快速构建分子复杂性方法[J]. 化学学报, 2016, 74(1): 54-60. |
| [5] | 汪学彬, 王晓丽, 胡静, 王兆亚, Pimpalpalle Tukaram M, Linker Torsten, 尹健. 新型糖氨基酸类化合物的合成研究[J]. 化学学报, 2015, 73(7): 699-704. |
| [6] | 胡齐, 李玉祥, 王静媛, 李亚鹏. pH敏感型聚合物PEG-b-PHEMA(His)的合成及胶束性能研究[J]. 化学学报, 2015, 73(5): 416-422. |
| [7] | 刘晓云, 郭颂, 武钰铃, 苗艳勤, 杜晓刚, 周禾丰, 王华, 郭鹍鹏. 核壳型Alq3@SiO2的一锅法制备[J]. 化学学报, 2013, 71(07): 1017-1021. |
| [8] | 程春生, 秦福涛, 魏振云, 任忠宝, 明旭. 氟吗啉合成工艺热危险性及动力学研究[J]. 化学学报, 2012, 70(10): 1227-1231. |
| [9] | 方云 吴梦洁 任月萍 江明. PVP-SDS软模板引导常温水相一锅法合成银纳米棒[J]. 化学学报, 2011, 69(15): 1737-1742. |
| [10] | 胡 瑾 方 云 王 婷 任月萍 陈方博 石 茵. 以SDS-PVP项链状软团簇为模板简易一锅法合成Cu2O中空亚微球[J]. 化学学报, 2009, 67(14): 1591-1596. |
| [11] | 胡艾希 董敏宇 谢艳丽 曹 高 叶 姣. 萘普生2-芳基吗啉乙酯的合成及其对环氧合酶-2的抑制活性[J]. 化学学报, 2008, 66(22): 2553-2557. |
| [12] | . 新型氮二烷基吗啉盐酸盐离子液体的合成及其性能研究[J]. 化学学报, 2008, 66(20): 2289-2294. |
| [13] | 施栩翎,杨镇军,韩梅,蔡孟深,程铁明. 11,2,4-三嗪类化合物的研究XXⅢ.6-氮杂嘌呤衍的合物[J]. 化学学报, 1995, 53(2): 199-204. |
| [14] | 康国钧,章本礼,卓金聪,高振衡,王如骥,王宏根. 烯氨酮晶体结构研究[J]. 化学学报, 1988, 46(2): 103-107. |
| [15] | 汪猷,徐耀忠,杨再完,刘象元,王绮文. 核糖核苷酸和核糖核苷的N-羧酰咪唑选样性酰化[J]. 化学学报, 1988, 46(12): 1195-1200. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||