化学学报 ›› 2026, Vol. 84 ›› Issue (1): 73-85.DOI: 10.6023/A25040118 上一篇 下一篇
研究论文
投稿日期:2025-04-14
发布日期:2025-08-26
Yichun Loua, Chengli Hea,b, Linrui Wanga, Xiaoli Cuia,*( )
)
Received:2025-04-14
Published:2025-08-26
Contact:
* E-mail: xiaolicui@fudan.edu.cn; Tel.: 13817061363
文章分享
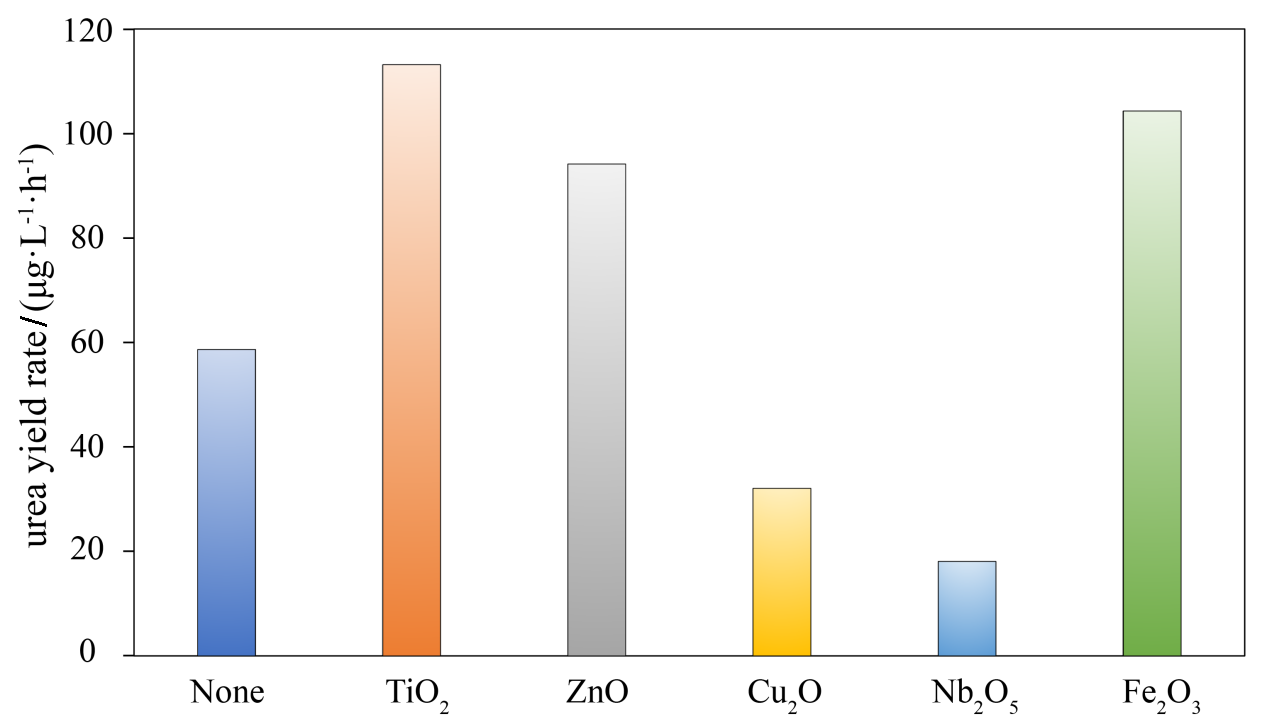
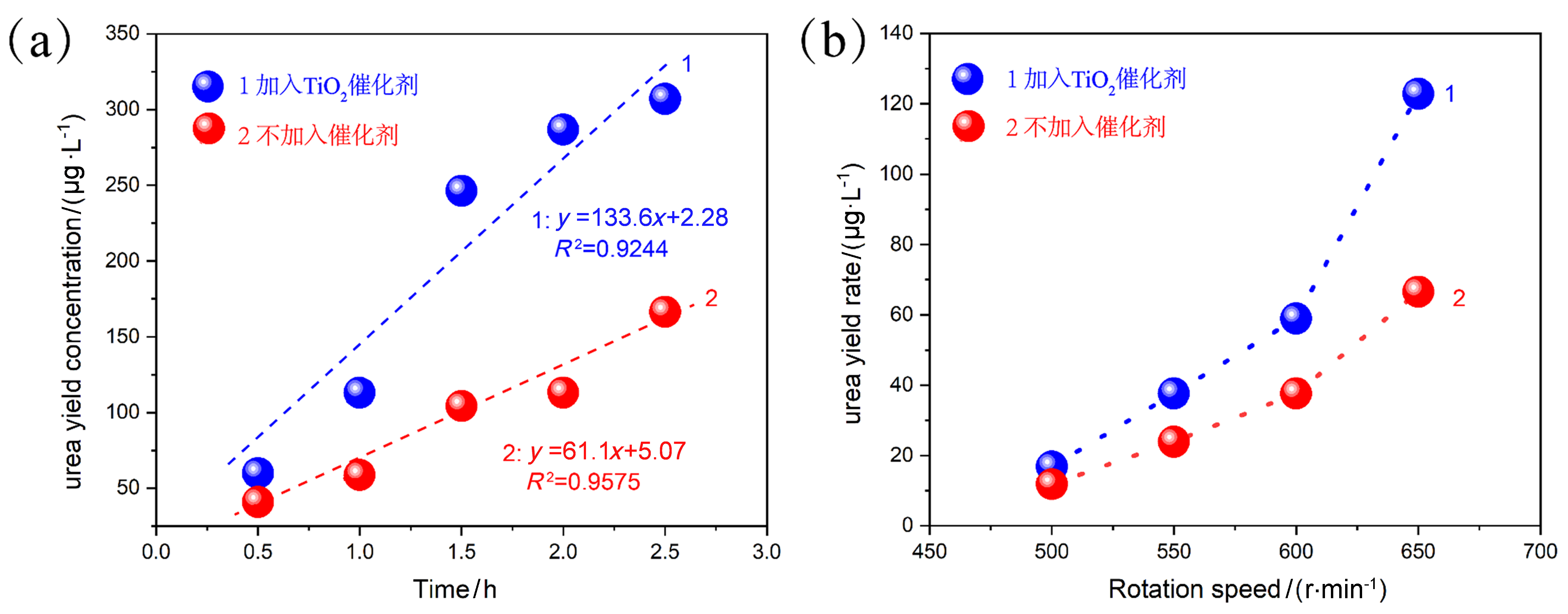
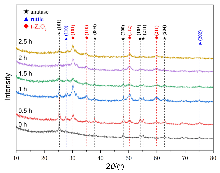
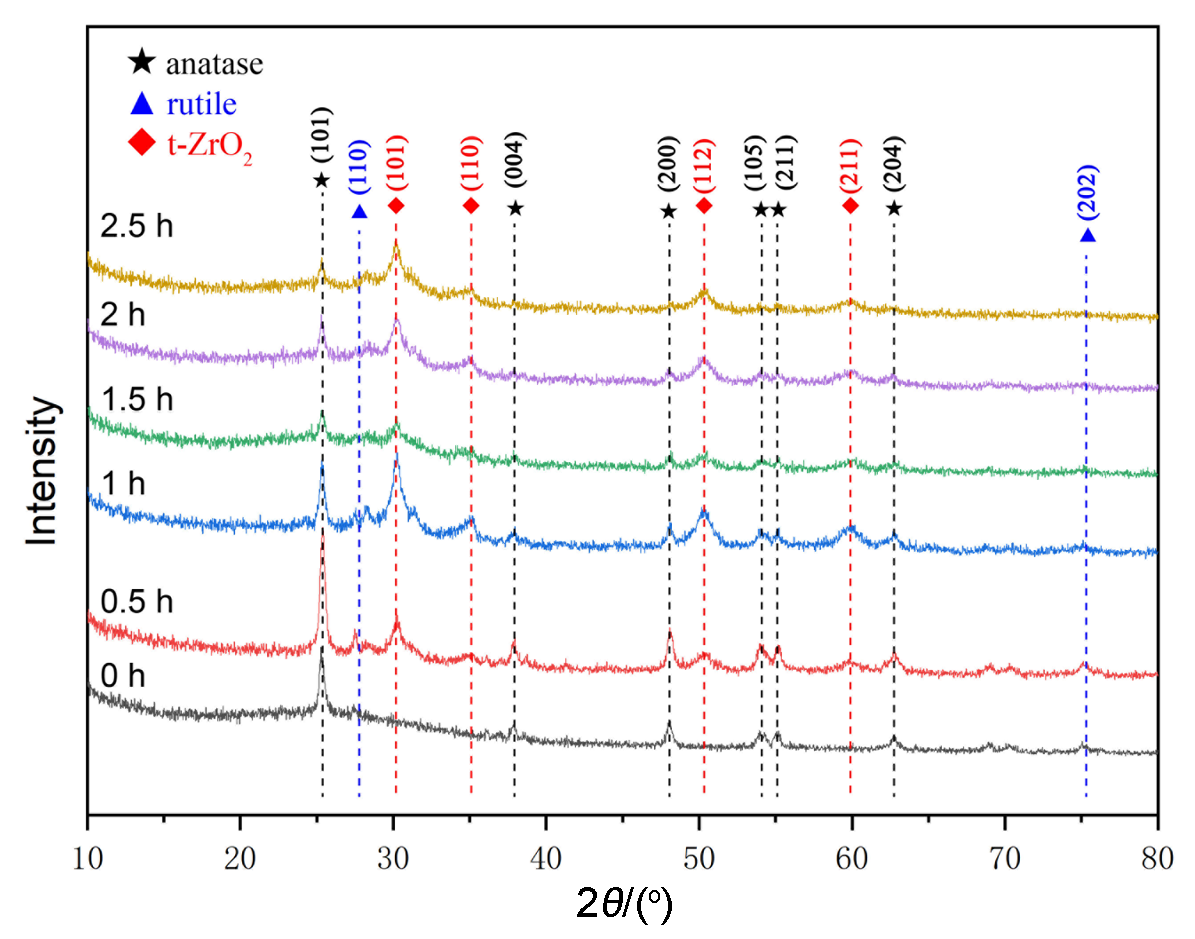
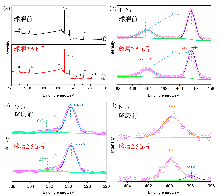
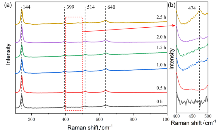
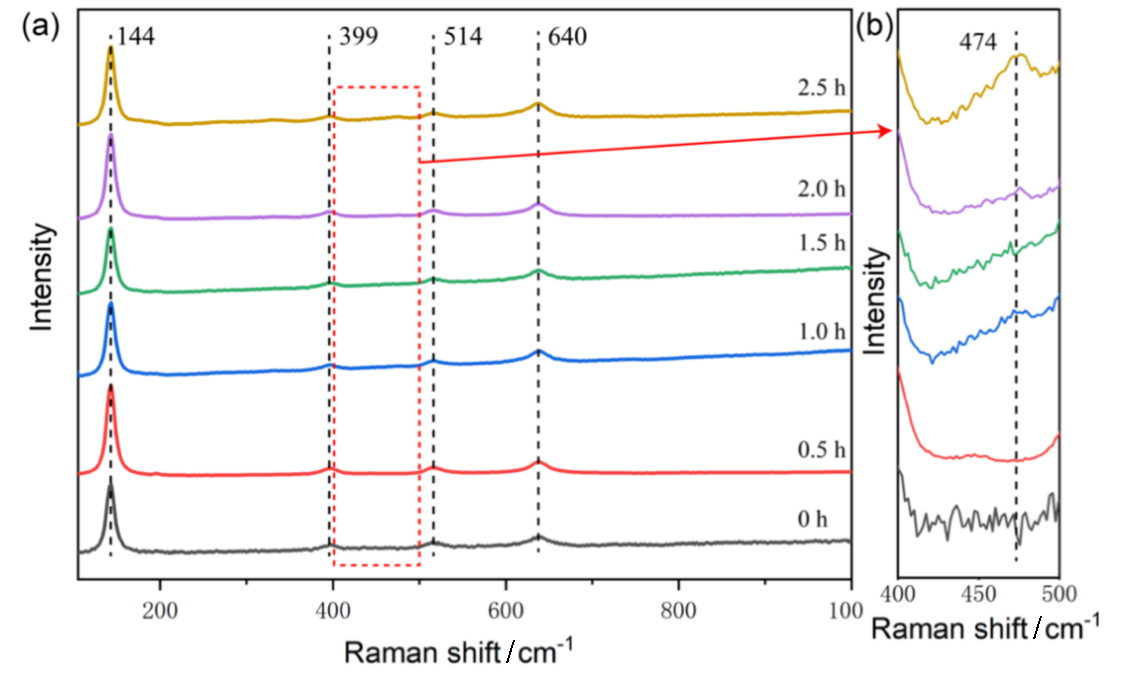
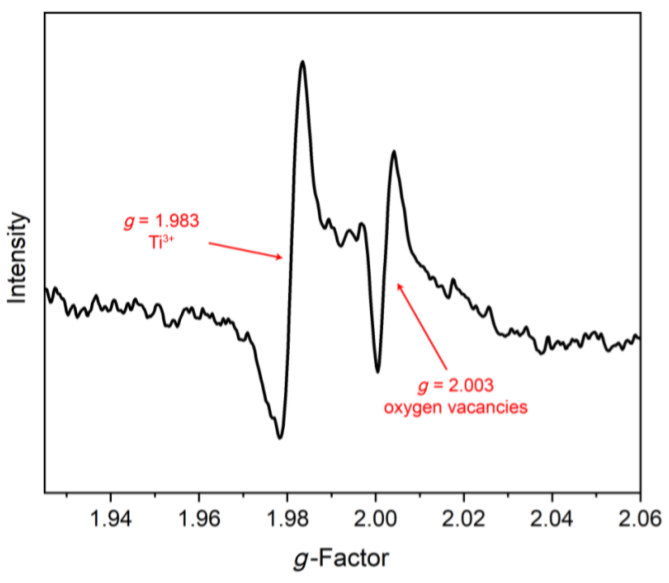
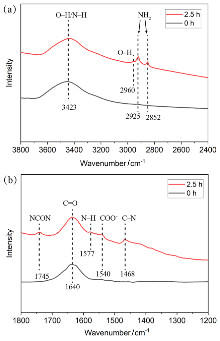
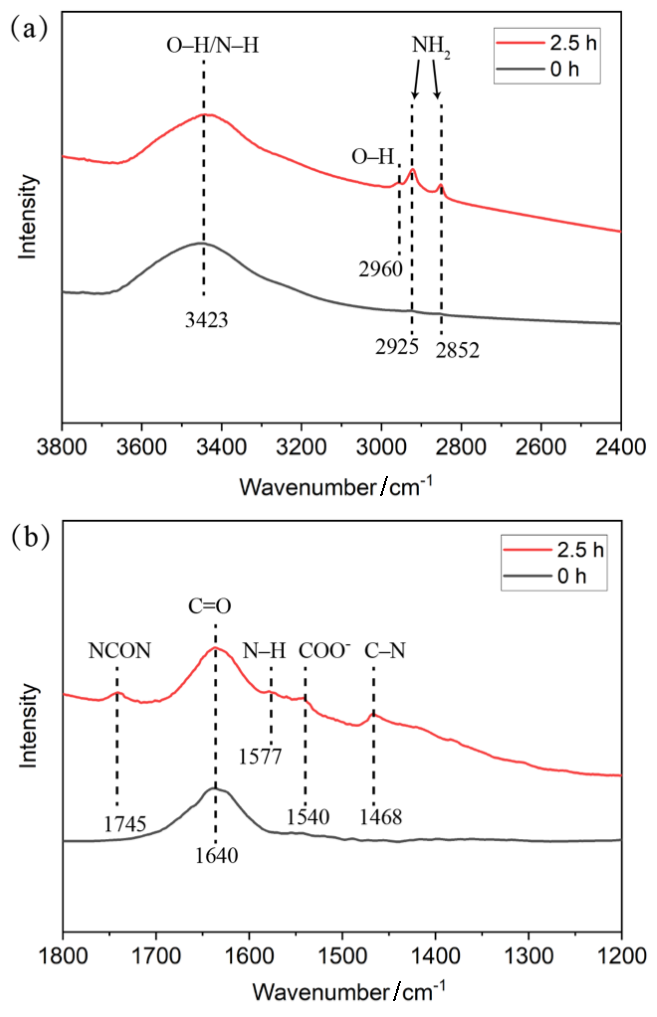
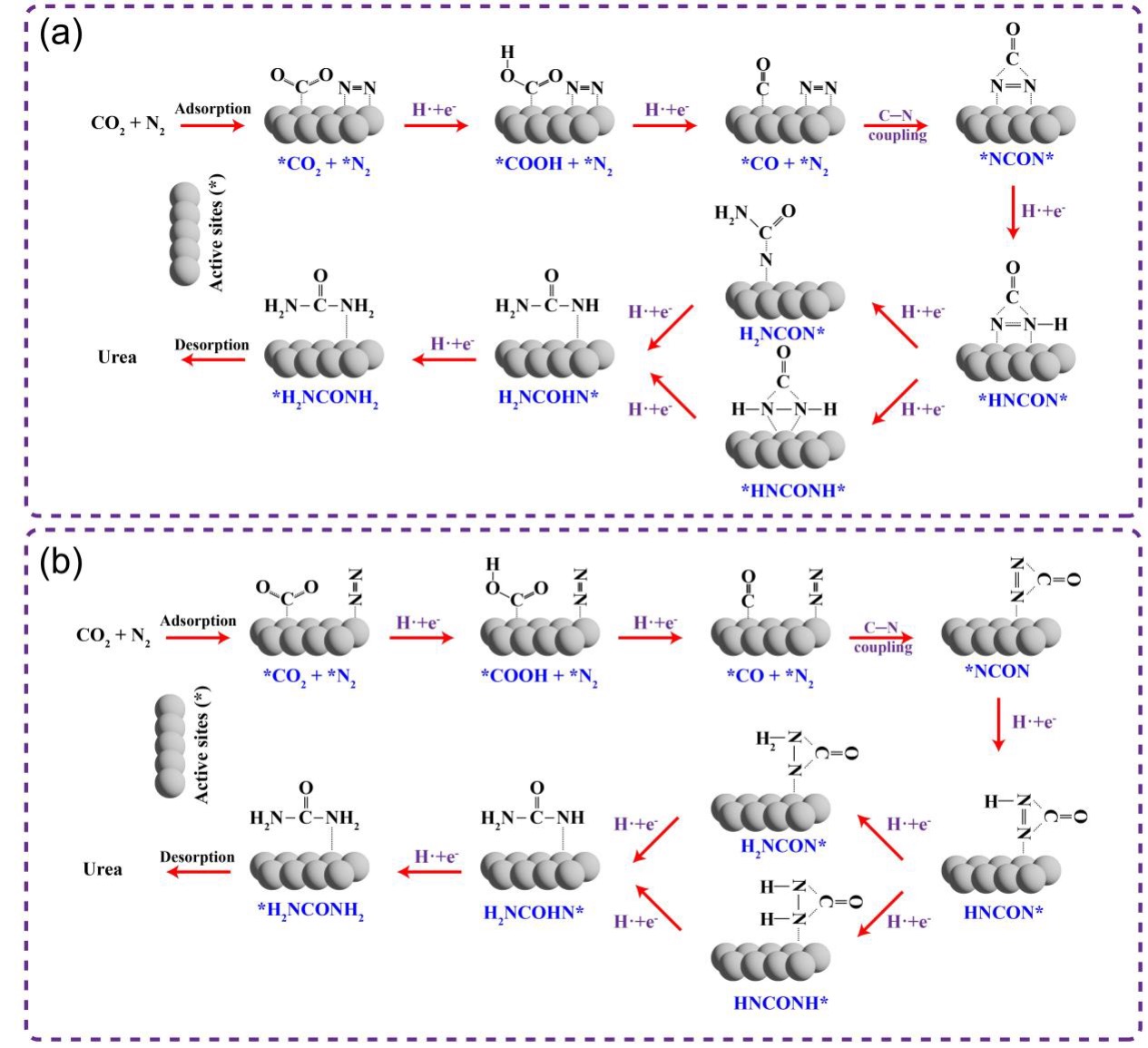
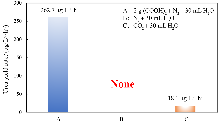
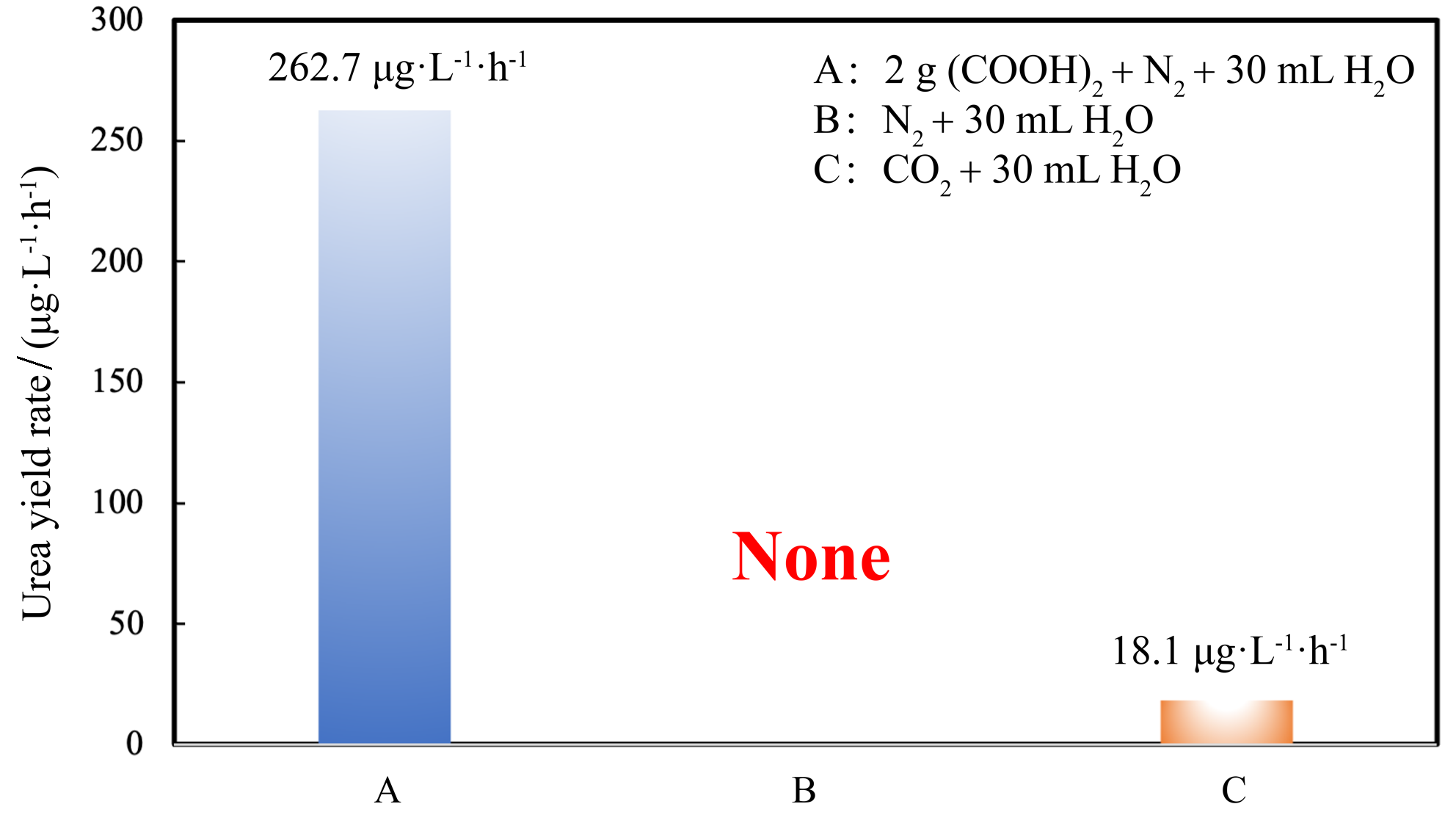
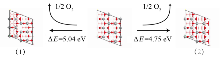
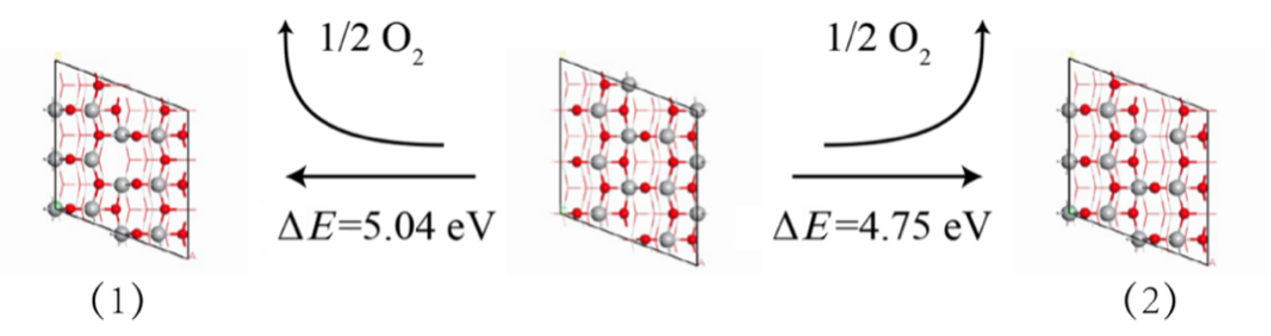
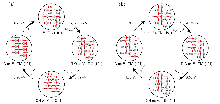
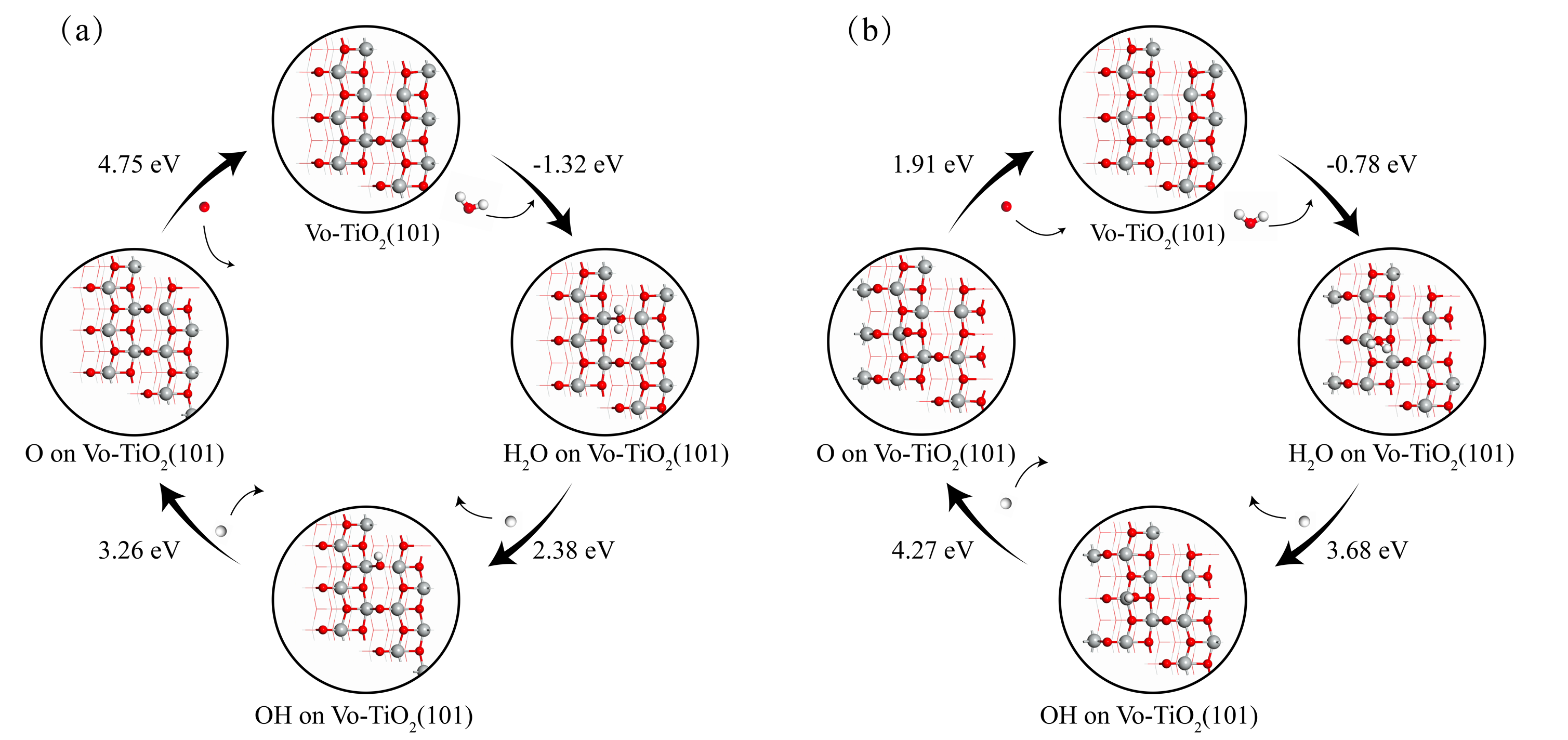
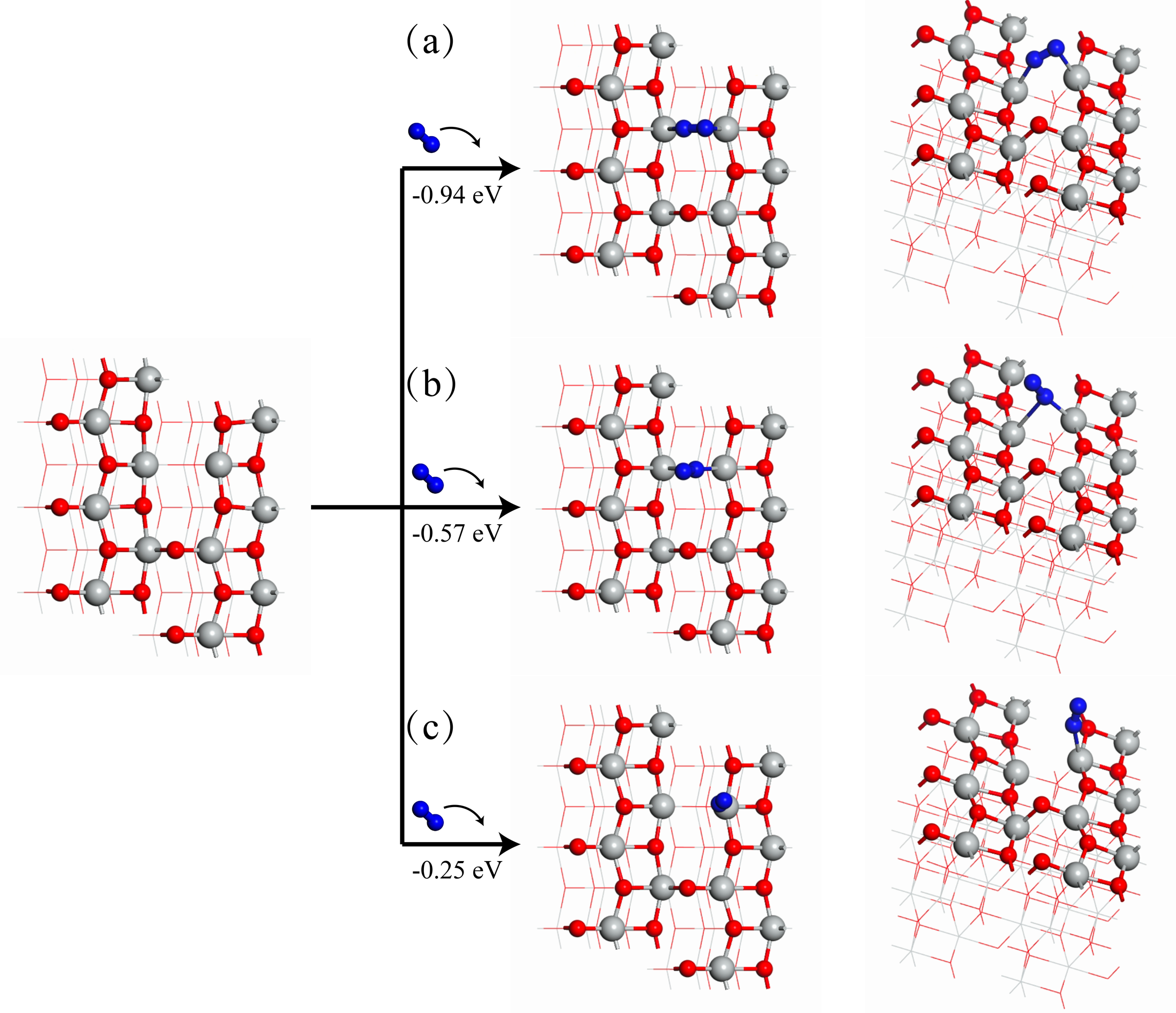
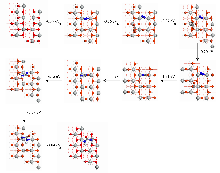
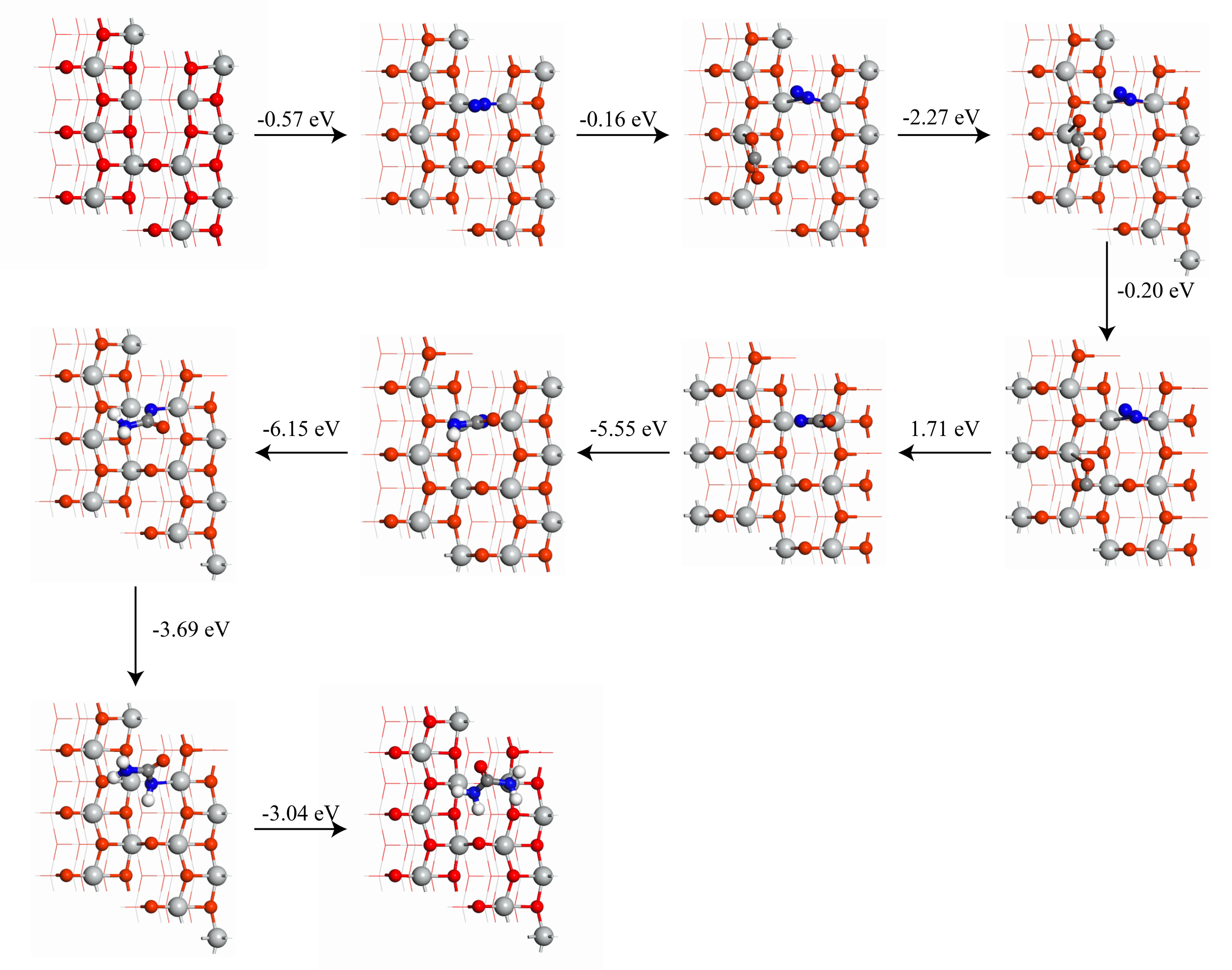
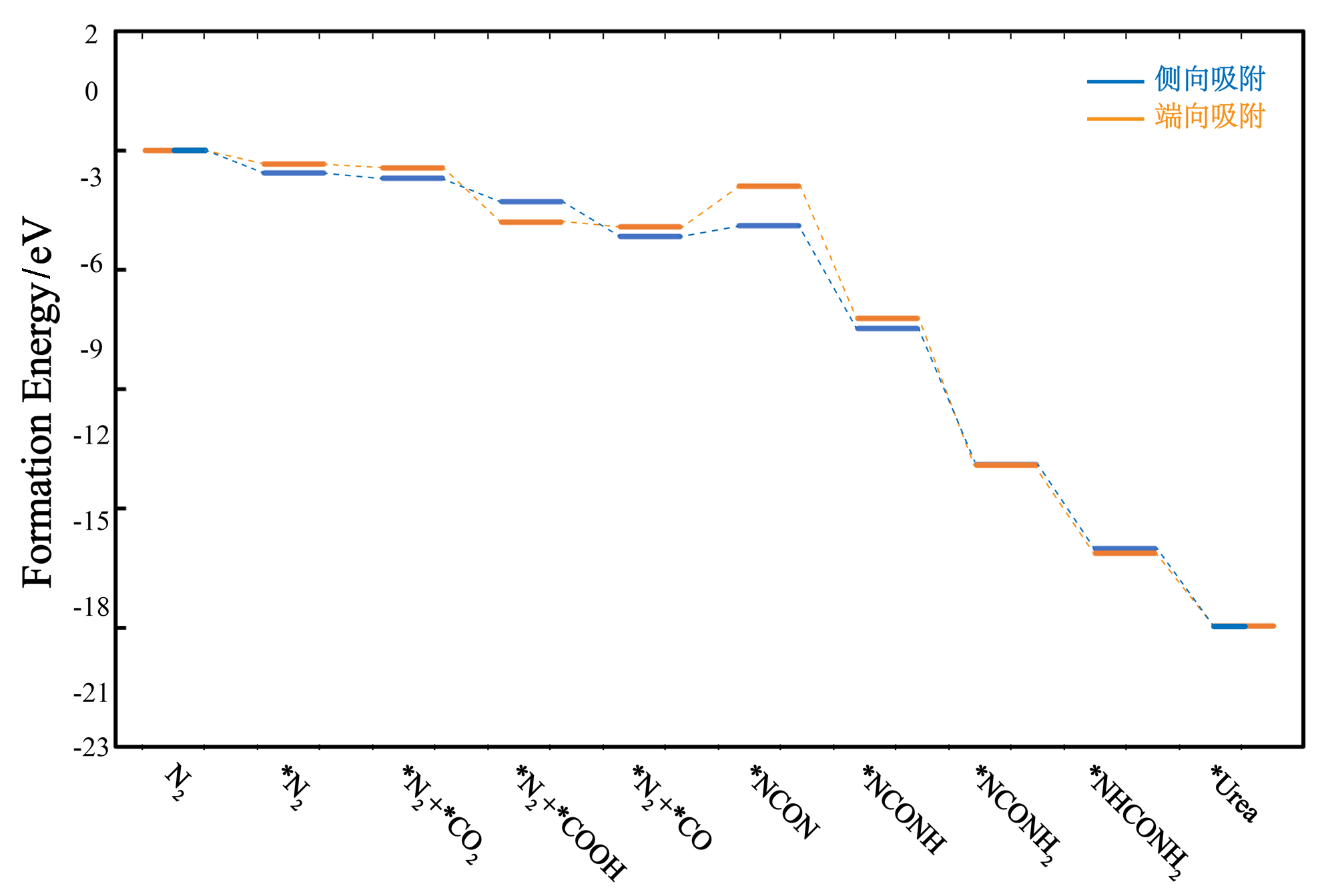
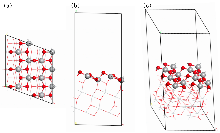
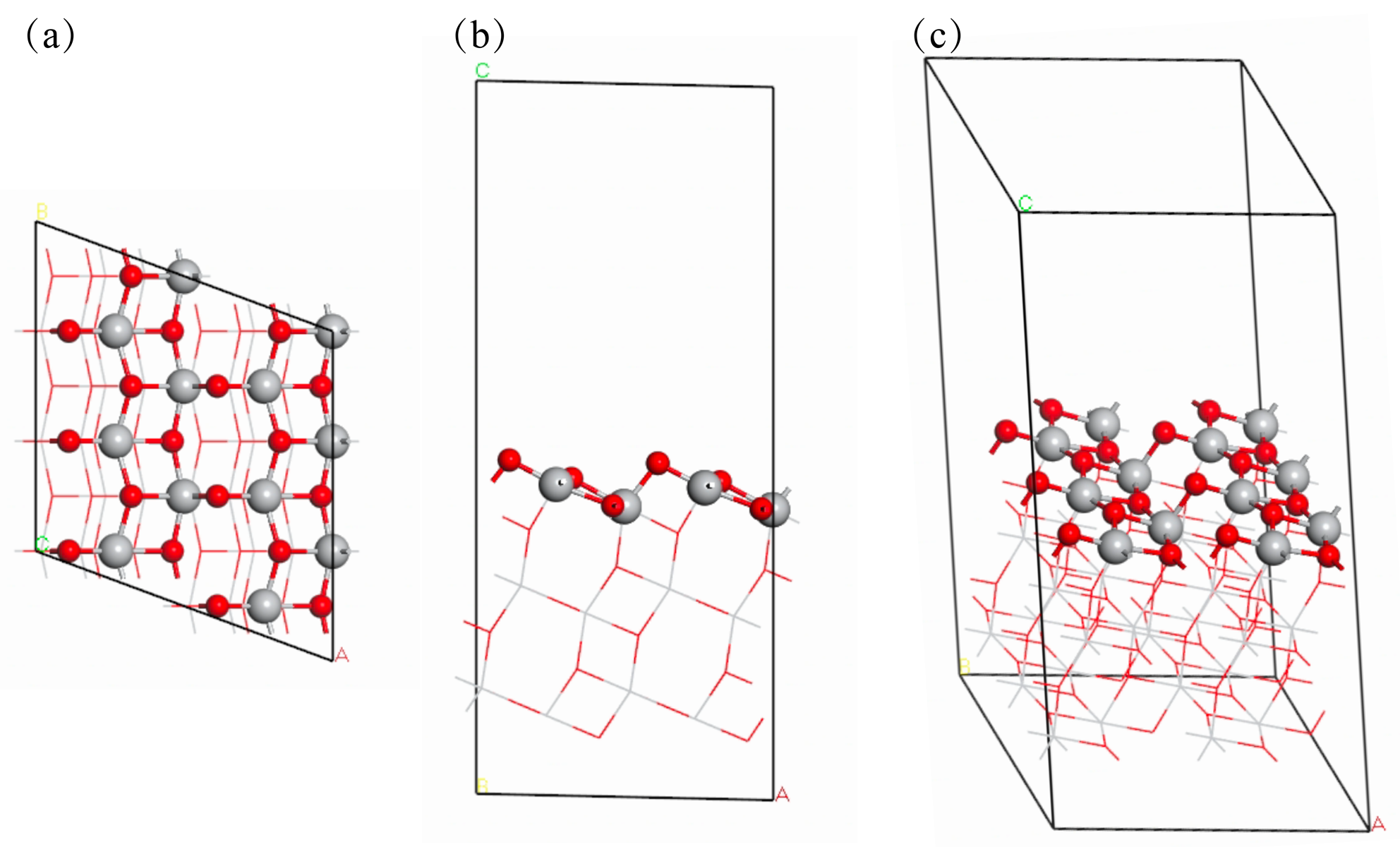
作为重要的化学品, 尿素的应用广泛, 然而传统Bosch-Meiser尿素生产工艺存在高能耗、高排放问题. 本工作提出在常温常压的条件下, 以N2、CO2和H2O为原料, 通过机械化学合成尿素的新策略. 利用二氧化锆球磨罐和磨球, 分别研究了TiO2、ZnO、Cu2O、Nb2O5、Fe2O3作为催化剂的机械化学合成尿素的催化作用. 其中TiO2表现出最佳的机械催化活性, 加入TiO2在相同条件下尿素的产率能够达到133.59 μg•L-1•h-1, 相对于无催化剂提升了2.2倍. 利用透射电子显微镜(TEM)、能量色散X射线能谱(EDS)、X射线衍射(XRD)、X射线光电子能谱(XPS)、电子顺磁共振(EPR)、Raman光谱对机械球磨合成尿素后的TiO2进行表征, 发现在机械球磨后TiO2中氧空位的含量上升. 利用傅里叶变换红外光谱(FT-IR)等技术检测了TiO2表面吸附的残余基团, 提出了可能的反应机理. 通过密度泛函理论计算研究了反应过程, H2O在催化剂表面的裂解和N2的活化是机械球磨合成尿素过程中两个重要步骤. TiO2中的氧空位不但能够促进对N2吸附和活化, 而且有助于H2O的裂解, 以释放出自由H. 在尿素合成的反应过程中, H2O裂解和*N2与*CO之间发生的C—N偶联反应是尿素合成过程中的共速控步. 本工作提出了一种以N2、CO2和H2O为原料通过机械化学合成尿素的方法, 初步揭示了TiO2对机械化学合成尿素的催化作用机理.
楼一淳, 何承溧, 王霖锐, 崔晓莉. 常温常压下N2、CO2和H2O体系TiO2机械化学合成尿素的实验与理论研究[J]. 化学学报, 2026, 84(1): 73-85.
Yichun Lou, Chengli He, Linrui Wang, Xiaoli Cui. Mechanochemical Urea Synthesis Using Nitrogen, Water and Carbon Dioxide with TiO2 under Mild Conditions: An Experimental and Theoretical Study[J]. Acta Chimica Sinica, 2026, 84(1): 73-85.





| [1] |
doi: 10.1021/acs.jpclett.4c03298 |
| [2] |
doi: 10.1016/j.joule.2024.04.004 |
| [3] |
doi: 10.1002/anie.v63.23 |
| [4] |
doi: 10.1038/ngeo325 |
| [5] |
doi: 10.1016/j.egypro.2014.11.834 |
| [6] |
doi: 10.1002/ente.v4.11 |
| [7] |
doi: 10.1038/ncomms4218 |
| [8] |
doi: 10.1021/acssuschemeng.4c05811 |
| [9] |
doi: 10.1016/j.ijhydene.2013.09.054 |
| [10] |
doi: 10.1002/adma.v29.33 |
| [11] |
doi: 10.1002/anie.v56.10 |
| [12] |
doi: 10.1002/adma.v29.17 |
| [13] |
doi: 10.1002/adma.v29.3 |
| [14] |
doi: 10.1021/jacs.7b04393 |
| [15] |
doi: 10.1039/D0MH00081G |
| [16] |
doi: 10.1039/C1CS15171A |
| [17] |
|
| [18] |
doi: 10.6023/cjoc202411009 |
|
(许秋玲, 孙瑞芬, 王均亮, 何晓山, 有机化学, 2025, 45, 2416.)
doi: 10.6023/cjoc202411009 |
|
| [19] |
doi: 10.1002/adma.v34.46 |
| [20] |
|
| [21] |
doi: 10.6023/A24090273 |
|
(刘雨涵, 高盼, 化学学报, 2024, 82, 1114.)
doi: 10.6023/A24090273 |
|
| [22] |
doi: 10.6023/cjoc202300062 |
|
(王官武, 潘虹, 有机化学, 2023, 43, 4010.)
doi: 10.6023/cjoc202300062 |
|
| [23] |
|
|
(冯浩洋, 邵晓阳, 王振华, 潘翔城, 科学通报, 2024, 69, 3412.)
|
|
| [24] |
doi: 10.1038/s41565-020-00809-9 |
| [25] |
doi: 10.1038/s41467-023-38050-2 |
| [26] |
doi: 10.1038/s41467-025-60715-3 |
| [27] |
doi: 10.1021/acssuschemeng.1c05643 |
| [28] |
|
| [29] |
|
| [30] |
doi: 10.1038/s41467-019-10888-5 pmid: 31253834 |
| [31] |
doi: 10.1016/j.apcatb.2017.01.025 |
| [32] |
doi: 10.1021/acs.analchem.9b05156 |
| [33] |
doi: 10.1126/science.1061051 pmid: 11452117 |
| [34] |
doi: 10.1016/j.seppur.2021.119287 |
| [35] |
doi: 10.1016/j.jcis.2021.11.180 |
| [36] |
doi: 10.1021/acs.jpcc.9b06450 |
| [37] |
doi: 10.3390/molecules27249032 |
| [38] |
doi: 10.1021/jp0273934 |
| [39] |
doi: 10.1021/j100267a018 |
| [40] |
doi: 10.1039/D2TA00661H |
| [41] |
|
| [42] |
doi: 10.3762/bjnano.6.86 |
| [43] |
doi: 10.1016/j.jcat.2010.07.033 |
| [44] |
doi: 10.1021/acs.nanolett.4c03451 |
| [45] |
doi: 10.1002/anie.v63.48 |
| [46] |
doi: 10.1021/jacs.7b12101 pmid: 29320173 |
| [47] |
doi: 10.1021/ma00162a008 |
| [48] |
doi: 10.1002/anie.v61.1 |
| [49] |
doi: 10.1039/D1RA07060F |
| [50] |
doi: 10.1002/anie.v62.33 |
| [51] |
doi: 10.1002/anie.v62.43 |
| [52] |
(a)
|
|
(b)
doi: 10.1016/j.apcatb.2023.123146 |
|
| [53] |
doi: 10.1038/s41467-021-24400-5 |
| [54] |
doi: 10.1021/acsenergylett.0c01895 |
| [55] |
doi: 10.1016/j.cej.2020.126033 |
| [56] |
doi: 10.1038/s41557-020-0481-9 pmid: 32541948 |
| [57] |
|
| [1] | 姚志豪, 张巍, 周昭仪, 李丹聪, 张凯凯, 刘涛, 胡文凯, 程守安, 胡铭轩, 刘昱佳. Sr/Fe协同调控LaMnO3电子结构及CO选择性催化还原反应机理研究[J]. 化学学报, 2026, 84(1): 30-42. |
| [2] | 王鑫, 史燚威, 杨瑞杰, 宋志国, 王敏. 含苯磺酸类配体的单核Cd(II)配合物催化无溶剂“一锅法”Biginelli反应[J]. 化学学报, 2025, 83(7): 674-684. |
| [3] | 卢一林, 董盛杰, 崔方超, 薄婷婷, 毛卓. 希托夫紫磷烯/SnS2范德华异质结作为直接全解水光催化剂的理论构建[J]. 化学学报, 2025, 83(4): 377-389. |
| [4] | 陈铭晖, 张博心, 魏滔, 孙兆雪, 冯亚青, 张宝. 三嗪共价骨架材料的层间位错行为及其光生载流子动力学理论研究[J]. 化学学报, 2025, 83(2): 93-100. |
| [5] | 张乃心, 石伟群, 王聪芝. 二价锕系配合物AnB8的理论研究[J]. 化学学报, 2025, 83(12): 1523-1529. |
| [6] | 马莹, 陈维希, 刘羽辰, 刘子义, 吴涛, 陆安慧, 王东琪. 六方氮化硼氧化模式的密度泛函理论研究[J]. 化学学报, 2025, 83(1): 52-59. |
| [7] | 王治业, 肖博怀. 利用平面σ-芳香性增强电子输运能力[J]. 化学学报, 2024, 82(5): 520-526. |
| [8] | 赵雨晴, 梁栋, 贾吉慧, 余荣民, 卢灿忠. 具有双吸电子基团D-A型配体的Ag(I)发光配合物的合成与性能研究[J]. 化学学报, 2024, 82(5): 486-492. |
| [9] | 赵玉强, 张霞, 杨芸如, 朱立平, 周莹. 聚集诱导发射光笼分子的设计合成及原位光激活成像研究[J]. 化学学报, 2024, 82(3): 265-273. |
| [10] | 黄广龙, 薛小松. “陈试剂”作为三氟甲基源机理的理论研究[J]. 化学学报, 2024, 82(2): 132-137. |
| [11] | 刘雨涵, 高盼. 使用机械化学生成的钙基重格氏试剂(R-CaX)对有机卤化物进行直接硼化[J]. 化学学报, 2024, 82(11): 1114-1119. |
| [12] | 黄伊晨, 聂长明, 王聪芝, 陈树森, 宋艳, 李昊, 石伟群. 羟基和氨基取代偕胺肟用于海水提铀的理论研究[J]. 化学学报, 2024, 82(10): 1050-1057. |
| [13] | 梁雪峰, 荆剑, 冯昕, 赵勇泽, 唐新员, 何燕, 张立胜, 李慧芳. 共价有机框架COF66/COF366的电子结构: 从单体到二维平面聚合物[J]. 化学学报, 2023, 81(7): 717-724. |
| [14] | 罗楚文, 孔超颖, 汤朝晖. 超声化学在生物医学中应用的研究进展★[J]. 化学学报, 2023, 81(7): 836-842. |
| [15] | 杨磊, 葛娇阳, 王访丽, 吴汪洋, 郑宗祥, 曹洪涛, 王洲, 冉雪芹, 解令海. 一种基于芴的大环结构的有效降低内重组能的理论研究[J]. 化学学报, 2023, 81(6): 613-619. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||