化学学报 ›› 2011, Vol. 69 ›› Issue (14): 1715-1720. 上一篇 下一篇
研究论文
袁江1,2,周惦武*,1,彭平1,侯德政2
Yuan Jiang1,2 Zhou Dianwu*,1 Peng Ping1 Hou Dezheng2
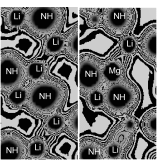
采用基于密度泛函理论的第一原理赝势平面波方法, 计算了LiNH2-X (X=Mg, Al, Ti, Nb)体系的晶体与电子结构及稳定性能. 负合金形成热与H原子解离能的计算发现: 合金化元素X在LiNH2中少量置换固溶时, 体系结构稳定性发生变化, 合金化增强了体系的解氢能力, 其中Nb提高LiNH2体系解氢效果最好, 理论计算预测合金化提高体系解氢性能与他人结果一致. 电子态密度(DOS)与电子密度分析发现: X (X=Mg, Al, Ti, Nb)合金化提高LiNH2解氢能力的主要原因是X导致LiNH2体系Fermi能级附近能隙值发生变化以及Li与NH之间的成键作用减弱.
中图分类号: