化学学报 ›› 2011, Vol. 69 ›› Issue (19): 2287-2292. 上一篇 下一篇
研究论文
张强*,许娟
ZHANG Qiang, XU Juan
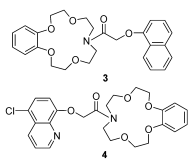
合成了萘基臂式苯并氮杂15-冠-5 (3)和5-氯喹啉基臂式苯并氮杂15-冠-5 (4)两种荧光化学传感器, 由元素分析、NMR等进行了结构表征|在乙醇溶剂中采用紫外光谱和荧光光谱法分别研究了冠醚3和4对金属离子K+, Ca2+, Mg2+, Pb2+, Co2+, Ni2+, Mn2+, Cu2+, Zn2+, Cd2+和Hg2+的选择性传感性能. 结果表明, 冠醚3仅对Cu2+离子有显著的传感作用, 是一种典型的辐射能量转移过程. 冠醚4对Ni2+, Co2+, Cu2+和Mn2+离子有特殊的敏感性, 金属离子浓度逐渐增大使冠醚4产生先增强后猝灭的荧光现象, 荧光增强归因于阻断了从冠环氮原子到喹啉荧光体的弱光诱导电子转移(PET)过程, 而选择性猝灭源于冠醚环与喹啉基团对金属离子协同螯合的机制. 冠醚3和4的构性关系表明, 发光体基团对传感器的选择性能够产生重要的影响.
中图分类号: