化学学报 ›› 2012, Vol. 70 ›› Issue (17): 1805-1811.DOI: 10.6023/A12060300 上一篇 下一篇
研究论文
柴云峰, 甘世凤, 潘远江
Chai Yunfeng, Gan Shifeng, Pan Yuanjiang
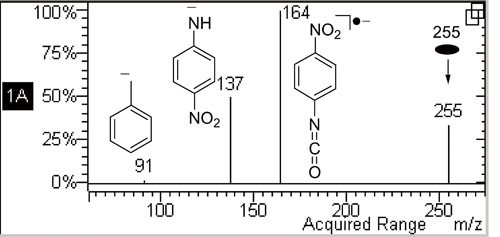
电喷雾串联质谱中偶电子负离子裂解产生阴离子自由基是一种违反偶电子规则的异常碎裂反应, 但是这种碎裂反应也常常被观察到, 其机理传统上一直被认为是共价键的简单均裂. 针对苯乙酰苯胺及其衍生物(R1C6H4CH2CONH- C6H4R2)的去质子化离子([M-H]-)裂解生成阴离子自由基这一特殊的碎裂反应提出了一个新颖的反应机理, 即离子/中性复合物介导的单电子转移反应机理. 以化合物3 (R1=H, R2=NO2)为模型提出的反应机理为, 首先氮上负电荷诱导CH2—CO键异裂生成[苄基负离子/4-硝基苯异氰酸酯]复合物中间体, 然后复合物中发生单电子转移反应产生4-硝基苯异氰酸酯阴离子自由基. 通过取代基效应研究(电子亲和势分析)、与文献报道的双分子电子转移反应比较和密度泛函理论计算等方法, 新反应机理得到了证明.