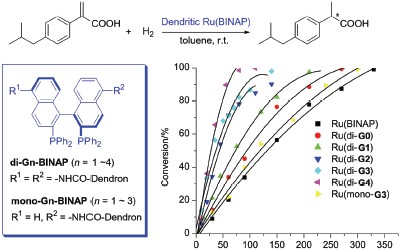
| [1] For selected reviews, see: (a) Oosterom, G. E.; Reek, J. N. H.; Kamer, P. C. J.; van Leeuwen, P. W. N. M. Angew. Chem., Int. Ed. 2001, 40, 1828; (b) Astruc, D.; Chardac, F. Chem. Rev. 2001, 101, 2991; (c) van Heerbeek, R.; Kamer, P. C. J.; van Leeuwen, P. W. N. M.; Reek, J. N. H. Chem. Rev. 2002, 102, 3717; (d) Fan, Q.-H.; Li, Y.-M.; Chan, A. S. C. Chem. Rev. 2002, 102, 3385. [2] Knapen, J. W. J.; van der Made, A. W.; de Wilde, J. C.; van Leeuwen, P. W. N. M.; Wijkens, P.; Grove, D. M.; van Koten, G. Nature 1994, 372, 659. [3] For selected reviews on chiral dendritic catalysts, see: (a) Seebach, D.; Rheiner, P. B.; Greiveldinger, G.; Butz, T.; Sellner, H. Top. Curr. Chem. 1998, 197, 125; (b) Kassube, J. K.; Gade, L. H. Top. Organomet. Chem. 2006, 20, 61; (c) Fan, Q.-H.; Deng, G.-J.; Feng, Y.; He, Y.-M. In Handbook of Asymmetric Heterogeneous Catalysis, Eds.: Ding, K.; Uozumi, Y., Wiley-VCH, Weinheim, 2008, p. 131; (d) Caminade, A. M.; Servin, P.; Laurent, R.; Majoral, J. P. Chem. Soc. Rev. 2008, 37, 56; (e) Ma, B.-D.; Fan, Q.-H. Sci. Sinica Chim. 2010, 40, 827. [4] For reviews on dendritic effect in catalytic reactions, see: (a) Chow, H.-F.; Leung, C.-F.; Wang, G.; Yang, Y. Y. C. R. Chem. 2003, 6, 735; (b) Helms, B.; Fréchet, J. M. J. Adv. Synth. Catal. 2006, 348, 1125; (c) Fan, Q.-H.; Ding, K.-L. Top. Organomet. Chem. 2011, 36, 207. [5] For selected examples of chiral dendrimer catalysts with positive dendritic effect, see: (a) Breinbauer, R.; Jacobsen, E. N. Angew. Chem., Int. Ed. 2000, 39, 3604; (b) Ribourdouille, Y.; Engel, G. D.;Richard-Plouet, M.; Gade, L. H. Chem. Commun. 2003, 1228; (c) Fan, Q.-H.; Chen, Y.-M.; Chen, X.-M.; Jiang, D.-Z.; Xi, F.; Chan, A. S. C. Chem. Commun. 2000, 789; (d) Wang, Z.-J.; Deng, G.-J.; Li, Y.; He, Y.-M.; Tang, W.-J.; Fan, Q.-H. Org. Lett. 2007, 9, 1243; (e) Zhang, F.; Li, Y.; Li, Z.-W.; He, Y.-M.; Zhu, S.-F.; Fan, Q.-H.; Zhou, Q.-L. Chem. Commun. 2008, 6048; (f) Mitsui, K.; Hyatt, S. A.; Turner, D. A.; Hadad, C. M.; Parquette, J. R. Chem. Commun. 2009, 3261; (g) Kehat, T.; Portnoy, M. Chem. Commun. 2007, 2823; (h) Kassube, J. K.; Wadepohl, H.; Gade, L. H. Adv. Synth. Catal. 2009, 351, 607; (i) Gissibl, A.; Padié, C.; Hager, M.; Jaroschik, F.; Rasappan, R.; Cuevas-Yaňez, E.; Turrin, C. O.; Caminade, A. M.; Majoral, J. P.; Reiser, O. Org. Lett. 2007, 9, 2895; (j) Garcia, L.;Roglans, A.; Laurent, R.; Majoral, J. P.; Pla-Quintana, A.; Caminade, A. M. Chem. Commun. 2012, 48, 9248. [6] (a) Deng, G.-J.; Fan, Q.-H.; Chen, X.-M. Chin. J. Chem. 2002, 20, 1139; (b) Deng, G.-J.; Fan, Q.-H.; Chen, X.-M.; Liu, D.-S.; Chan, A. S. C. Chem. Commun. 2002, 1570; (c) Deng, G.-J.; Fan, Q.-H.; Chen, X.-M.; Liu, G.-H. J. Mol. Catal. A 2003, 193, 21; (d) Yi, B.; Fan, Q.-H.; Deng, G.-J.; Li, Y.-M.; Qiu, L.-Q.; Chan, A. S. C. Org. Lett. 2004, 6, 1361; (e) Huang, Y.-Y.; He, Y.-M.; Zhou, H.-F.; Wu, L.; Li, B.-L.; Fan, Q. H. J. Org. Chem. 2006, 71, 2874; (f) Tang, W.-J.; Huang, Y.-Y.; He, Y.-M.; Fan, Q.-H. Tetrahedron: Asymmetry 2006, 17, 536; (g) Deng, G.-J.; Li, G.-R.; Zhu, L.-Y.; Zhou, H.-F.; He, Y.-M.; Fan, Q.-H.; Shuai, Z.-G. J. Mol. Catal. A 2006, 244, 118; (h) Li, Y.; He, Y.-M.; Li, Z.-W.; Zhang, F.; Fan, Q.-H. Org. Biomol. Chem. 2009, 7, 1890; (i) Liu, J.; Feng, Y.; Ma, B.-D.; He, Y.-M.; Fan, Q.-H. Eur. J. Org. Chem. 2012, 6737. [7] Fan, Q.-H.; Hu, W.-H.; Ren, C.-Y.; Yeung, C. H.; Chan, A. S. C. J. Am. Chem. Soc. 1999, 121, 7407. [8] Hawker, C. J.; Fréchet, J. M. J. J. Am. Chem. Soc. 1990, 112, 7638. [9] Harrington, P. J.; Lodewijk, E. Org. Process Res. Dev. 1997, 1, 72. [10] Ohta, T.; Takaya, H.; Kitamura, M.; Nagai, K.; Noyori, R. J. Org. Chem. 1987, 52, 3174. [11] Niu, Y.-H.; Yeung, L. K.; Crooks, R. M. J. Am. Chem. Soc. 2001, 123, 6840. [12] (a) Dang, T.-P.; Kagan, H. B. J. Chem. Soc., Chem. Commun. 1971, 481; (b) Miyashita, A.; Yasuda, A.; Takaya, H.; Toriumi, K.; Ito, T.; Souchi, T.; Noyori, R. J. Am. Chem. Soc. 1980, 102, 7932. |