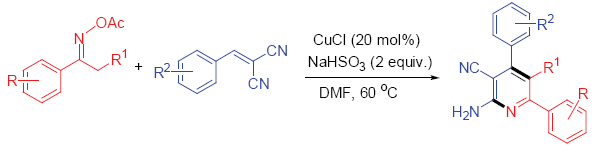
| [1] (a) Narasaka, K.; Kitamura, M. Eur. J. Org. Chem. 2005, 4505; (b) Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V. V.; Noodleman, L.; Sharpless, K. B.; Fokin, V. V. J. Am. Chem. Soc. 2005, 127, 210; (c) Alonso, D. A.; Nájera, C. Chem. Soc. Rev. 2010, 39, 2891; (d) Sukhorukov, A. Y.; Ioffe, S. L. Chem. Rev. 2011, 111, 5004; (e) Cai, H.; Li, D.; Liu, Z.; Wang, G. Acta Chim. Sinica 2013, 71, 717. (蔡海婷, 李丹丹, 刘姿, 王官武, 化学学报, 2013, 71, 717); (f) Ran, L.; Liang, H.; Guan, Z. Chin. J. Org. Chem. 2013, 33, 66. (冉陇飞, 梁浩, 关正辉, 有机化学, 2013, 33, 66); (g) Dai, H.; Yu, H.; Liu, J.; Qin, X.; Wang, T.; Zhang, X.; Qin, Z.; Fang, J. Chin. J. Org. Chem. 2013, 33, 1104. (戴红, 于海波, 刘建兵, 秦雪, 王婷婷, 张欣, 秦振芳, 方建新, 有机化学, 2013, 33, 1104); (h) Liu, Z.; Wei, W.; Gan, C.; Huang, Y.; Liu, S.; Zhou, M.; Cui, J. Chin. J. Org. Chem. 2013, 33, 2551. (刘志平, 韦万兴, 甘春芳, 黄燕敏, 刘盛, 周敏, 崔建国, 有机化学, 2013, 33, 2551). [2] (a) Ramalingan, C.; Park, Y.-T. J. Org. Chem. 2007, 72, 4536; (b) Hashimoto, M.; Obora, Y.; Sakaguchi, S.; Ishii, Y. J. Org. Chem. 2008, 73, 2894; (c) Ramón, R. S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S. P. J. Org. Chem. 2010, 75, 1197. [3] (a) Choi, E.; Lee, C.; Na, Y.; Chang, S. Org. Lett. 2002, 4, 2369; (b) De Luca, L.; Giacomelli, G.; Porcheddu, A. J. Org. Chem. 2002, 67, 6272; (c) Augustine, J. K.; Atta, R. N.; Ramappa, B. K.; Boodappa, C. Synlett 2009, 3378; (d) Zhu, J.-L.; Lee, F.-Y.; Wu, J.-D.; Kuo, C.-W.; Shia, K.-S. Synlett 2007, 1317. [4] (a) Tanaka, K.; Kitamura, M.; Narasaka, K. Bull. Chem. Soc. Jpn. 2005, 78, 1659; (b) Liu, S.; Yu, Y.; Libebeskind, L. S. Org. Lett. 2007, 9, 1947; (c) Liu, S.; Liebeskind, L. S. J. Am. Chem. Soc. 2008, 130, 6918; (e) Too, P. C.; Chua, S. H.; Wong, S. H.; Chiba, S. J. Org. Chem. 2011, 76, 6159. [5] (a) Ren, Z.-H.; Zhang, Z.-Y.; Yang, B.-Q.; Wang, Y.-Y.; Guan, Z.-H. Org. Lett. 2011, 13, 5394; (b) Ran, R.; Ren, Z.-H.; Wang, Y.-Y.; Guan, Z.-H. Green Chem. 2014, 16, 112. [6] (a) Tang, X.; Huang, L.; Qi, C.; Wu, W.; Jiang, H. Chem. Commun. 2013, 49, 9597; (b) Huang, H.; Ji, X.; Tang, X.; Zhang, M.; Li, X.; Jiang, H. Org. Lett. 2013, 15, 6254; (c) Tang, X.; Huang, L.; Xu, Y.; Yang, J.; Wu, W.; Jiang, H. Angew. Chem., Int. Ed. DOI: 10.1002/anie.201311217. [7] Wei, Y.; Yoshikai, N. J. Am. Chem. Soc. 2013, 135, 3756. [8] (a) Deng, J.; Sanchez, T.; Al-Mawsawi, L. Q.; Dayam, R.; Yunes, R. A.; Garofalo, A.; Bolger, M. B.; Neamati, N. Bioorg. Med. Chem. 2007, 15, 4985; (b) Fraley, A. W.; Chen, D.; Johnson, K.; McLaughlin, L. W. J. Am. Chem. Soc. 2003, 125, 616. [9] Eri?, S.; Ke, S.; Barata, T.; Solmajer, T.; Stankovi?, J. A.; Jurani?, Z.; Savi?a, V.; Zloh, M. Bioorg. Med. Chem. 2012, 20, 5220. [10] Laufer, S. A.; Zimmermann, W.; Ruff, K. J. J. Med. Chem. 2004, 47, 6311. [11] Ji, H.; Delker, S. L.; Li, H.; Martásek, P.; Roman, L. J.; Poulos, T. L.; Silverman, R. B. J. Med. Chem. 2010, 53, 7804. [12] Zong, R.; Zhou, H.; Thummel, R. P. J. Org. Chem. 2008, 73, 4334. [13] (a) Chen, J.; Pang, Q.; Sun, Y.; Li, X. J. Org. Chem. 2011, 76, 3523; (b) Chen, J.; Song, G.; Pan, C. L.; Li, X. Org. Lett. 2010, 12, 5426. [14] Bentabed-Ababsa, G.; Ely, S. C. S.; Hesse, S.; Nassar, E.; Chevallier, F.; Nguyen, T. T.; Derdour, A.; Mongin, F. J. Org. Chem. 2010, 75, 839. [15] (a) Hand, E. S.; Paudler, W. W. J. Org. Chem. 1978, 43, 2900; (b) Denora, N.; Laquintana, V.; Pisu, M. G.; Dore, R.; Murru, L.; Latrofa, A.; Trapani, G.; Sanna, E. J. Med. Chem. 2008, 51, 6876; (c) Yan, R.-L.; Yan, H.; Ma, C.; Ren, Z.-Y.; Gao, X.-A.; Huang, G.-S.; Liang, Y.-M. J. Org. Chem. 2012, 77, 2024; (d) Zeng, J. Y.; Tan, J.; Leow, M. L.; Liu, X.-W. Org. Lett. 2012, 14, 4386; (e) He, C.; Hao, J.; Xu, H.; Mo, Y.; Liu, H.; Han, J.; Lei, A. Chem. Commun. 2012, 48, 11073; (f) Santra, S.; Bagdi, A. K.; Majee, A.; Hajra, A. Adv. Synth. Catal. 2013, 355, 1065; (g) Bagdi, A. K.; Rahman, M.; Santra, S.; Majee, A.; Hajra, A. Adv. Synth. Catal. 2013, 355, 1741. [16] Chichibabin, A. E.; Zeide, O. A. J. Russ. Phys. Chem. Soc. 1914, 46, 1216. [17] (a) Cai, Z.-J.; Wang, S. Y.; Ji, S.-J. Org. Lett. 2012, 14, 6068; (b) Cai, Z.-J.; Wang, S. Y.; Ji, S.-J. Adv. Synth. Catal. 2013, 355, 2686. [18] Wu, Q.; Zhang, Y.; Cui, S. Org. Lett. 2014, 16, 1350.(a) Trost, B. M.; Rhee, Y. H. J. Am. Chem. Soc. 2002, 124, 2528; (b) Nitta, M.; Iino, Y. Bull. Chem. Soc. Jpn. 1986, 59, 2365; (c) Deeming, A. J.; Owen, D. W.; Powell, N. I. J. Organomet. Chem. 1990, 398, 299; (d) Guan, Z.-H.; Zhang, Z.-Y.; Ren, Z.-H.; Wang, Y.-Y.; Zhang, X. J. Org. Chem. 2011, 76, 339. |