化学学报 ›› 2019, Vol. 77 ›› Issue (9): 932-938.DOI: 10.6023/A19050169 上一篇
所属专题: 有机自由基化学
研究论文
许健ab, 张世樊c, 罗莹c, 张荔c, 张帆c, 黄挺菁c, 宋秋玲ab*( )
)
投稿日期:2019-05-11
发布日期:2019-08-12
通讯作者:
宋秋玲
E-mail:qsong@hqu.edu.cn
基金资助:
Xu, Jianab, Zhang, Shifanc, Luo, Yingc, Zhang, Lic, Zhang, Fanc, Huang, Tingjingc, Song, Qiulingab*( )
)
Received:2019-05-11
Published:2019-08-12
Contact:
Song, Qiuling
E-mail:qsong@hqu.edu.cn
Supported by:文章分享
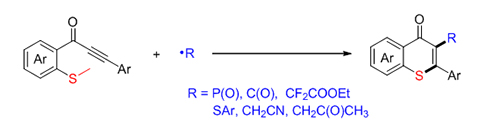
硫代吡喃酮是一种重要的结构骨架, 广泛存在于天然产物、潜在药物及生物活性分子中. 因此, 发展简洁、高效构建硫代吡喃酮的合成方法具有重要意义. 发展了一种自由基促进的硫甲基取代炔酮的加成环化反应来构建硫代吡喃酮环的新方法. 该方法具有底物适用性广, 一系列自由基前体如二苯基膦氧、硫酚、醛等都可以在该反应体系中实现转化. 机理研究表明, 自由基前体对炔酮的选择性加成得到C(sp 2)自由基中间体, 该中间体促进的C(sp 2)—S键构建及C(sp 3)—S键断裂是关键步骤.
许健, 张世樊, 罗莹, 张荔, 张帆, 黄挺菁, 宋秋玲. 自由基促进硫甲基取代的炔酮的环化反应[J]. 化学学报, 2019, 77(9): 932-938.
Xu, Jian, Zhang, Shifan, Luo, Ying, Zhang, Li, Zhang, Fan, Huang, Tingjing, Song, Qiuling. Radical Promoted Annulation of Alkynones for the Construction of 2,3-Disubstituted Thiochromones[J]. Acta Chimica Sinica, 2019, 77(9): 932-938.
| Entry | Catalyst (mol%) | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | AgNO3 (100) | CH3CN | 51 | |
| 2 | AgNO3 (20) | Mg(NO3)2?6H2O | CH3CN | 58 |
| 3 | AgOTf (20) | Mg(NO3)2?6H2O | CH3CN | 67 |
| 4 | Ag2CO3 (20) | Mg(NO3)2?6H2O | CH3CN | 80 |
| 5 | AgBF4 (20) | Mg(NO3)2?6H2O | CH3CN | 60 |
| 6 | Ag2O (20) | Mg(NO3)2?6H2O | CH3CN | 57 |
| 7 | AgOAc (20) | Mg(NO3)2?6H2O | CH3CN | 74 |
| 8 | Ag2CO3 (20) | Mg(NO3)2?6H2O | THF | 60 |
| 9 | Ag2CO3 (20) | Mg(NO3)2?6H2O | toluene | 65 |
| 10 | Ag2CO3 (20) | Mg(NO3)2?6H2O | DCE | 70 |
| 11 | Ag2CO3 (20) | Zn(NO3)2?6H2O | CH3CN | 84 |
| 12 | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 90 |
| 13c | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 81 |
| 14d | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 75 |
| Entry | Catalyst (mol%) | Additive | Solvent | Yieldb/% |
|---|---|---|---|---|
| 1 | AgNO3 (100) | CH3CN | 51 | |
| 2 | AgNO3 (20) | Mg(NO3)2?6H2O | CH3CN | 58 |
| 3 | AgOTf (20) | Mg(NO3)2?6H2O | CH3CN | 67 |
| 4 | Ag2CO3 (20) | Mg(NO3)2?6H2O | CH3CN | 80 |
| 5 | AgBF4 (20) | Mg(NO3)2?6H2O | CH3CN | 60 |
| 6 | Ag2O (20) | Mg(NO3)2?6H2O | CH3CN | 57 |
| 7 | AgOAc (20) | Mg(NO3)2?6H2O | CH3CN | 74 |
| 8 | Ag2CO3 (20) | Mg(NO3)2?6H2O | THF | 60 |
| 9 | Ag2CO3 (20) | Mg(NO3)2?6H2O | toluene | 65 |
| 10 | Ag2CO3 (20) | Mg(NO3)2?6H2O | DCE | 70 |
| 11 | Ag2CO3 (20) | Zn(NO3)2?6H2O | CH3CN | 84 |
| 12 | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 90 |
| 13c | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 81 |
| 14d | Ag2CO3 (10) | Zn(NO3)2?6H2O | CH3CN | 75 |
| Entrya | T/℃ | Oxidant (equiv.) | Yieldb/% |
|---|---|---|---|
| 1 | 100 | DTBP (2) | 54 |
| 2 | 100 | K2S2O8 (2) | Trace |
| 3 | 100 | Oxone (2) | Trace |
| 4 | 100 | TBHP (2) | 61 |
| 5 | 100 | BPO (2) | 60 |
| 6 | 100 | TBPB (2) | 75 |
| 7 | 100 | TBPB (1.5) | 70 |
| 8 | 100 | TBPB (2.5) | 72 |
| 9 | 120 | TBPB (2) | 90 |
| 10 | 80 | TBPB (2) | 54 |
| Entrya | T/℃ | Oxidant (equiv.) | Yieldb/% |
|---|---|---|---|
| 1 | 100 | DTBP (2) | 54 |
| 2 | 100 | K2S2O8 (2) | Trace |
| 3 | 100 | Oxone (2) | Trace |
| 4 | 100 | TBHP (2) | 61 |
| 5 | 100 | BPO (2) | 60 |
| 6 | 100 | TBPB (2) | 75 |
| 7 | 100 | TBPB (1.5) | 70 |
| 8 | 100 | TBPB (2.5) | 72 |
| 9 | 120 | TBPB (2) | 90 |
| 10 | 80 | TBPB (2) | 54 |
| [1] | (a) Nakazumi, H.; Ueyama, T.; Kitao, T . J. Heterocycl. Che. 1984, 21, 193. |
| (b) Couquelet, J.; Tronche, P.; Niviere, P.; Andraud, G . Trav. Soc. Pharm. Montpellie. 1963, 23, 214.. | |
| (c) Nakazumi, H.; Ueyama, T.; Kitao, T . J. Heterocycl. Chem. 1984, 2, 193. | |
| [2] | (a) Holshouser, M. H.; Loeffler, L. J.; Hall, I. H . J. Med. Che. 1981, 24, 853. |
| (b) Razdan, R. K.; Bruni, R. J.; Mehta, A. C.; Weinhardt, K. K.; Papanastassiou, Z. B . J. Med. Che. 1978, 21, 643. | |
| [3] |
Dhanak, D.; Keenan, R. M.; Burton, G.; Kaura, A.; Darcy, M. G. D.; Shah, H.; Ridgers, L. H.; Breen, A.; Lavery, P.; Tew, D. G.; West, A . Bioorg. Med. Chem. Let. 1998, 8, 3677.
doi: 10.1016/S0960-894X(98)00666-0 |
| [4] |
(a) Sangeetha, S.; Sekar, G . Org. Let. 2018, 21, 75.
doi: 10.1021/acs.orglett.8b03508 |
|
(b) Zhang, F.; Wu, X. F . J. Org. Che. 2018, 83, 13612.
doi: 10.1021/acs.orglett.8b03508 |
|
|
(c) Kim, H. Y.; Song, E.; Oh, K . Org. Lett. 2017, 19, 312.
doi: 10.1021/acs.orglett.8b03508 |
|
|
(d) Zhu, F.-X.; Wu, X.-F . J. Org. Chem. 2018, 83, 13612.
doi: 10.1021/acs.orglett.8b03508 |
|
| [5] |
(a) Schneller, S. W . Adv. Heterocycl. Chem. 1975, 18, 59.
doi: 10.1246/bcsj.63.847 |
|
(b) Nakazumi, H.; Wanatabe, S.; Kitaguchi, T.; Kitao, T . Bull. Chem. Soc. Jpn. 1990, 63, 847.
doi: 10.1246/bcsj.63.847 |
|
|
(c) Razdan, R. K.; Bruni, R. J.; Mehta, A. C.; Weinhardt, K. K.; Papanastassiou, Z. B . J. Med. Chem. 1978, 21, 643.
doi: 10.1246/bcsj.63.847 |
|
|
(d) Buggle, K.; Delahunty, J. J.; Philbin, E. M.; Ryan, N. D . J. Chem. Soc. C. 1971, 3168.
doi: 10.1246/bcsj.63.847 |
|
| [6] | Zhou, C.; Dubrovsky, A. V.; Larock, R. C . J. Org. Che. 2006, 71, 1626. |
| [7] |
Willy, B.; Frank, W.; Müller, T. J . J. Org. Biomol. Chem. 2010, 8, 90.
doi: 10.1039/B917627F |
| [8] | Yang, X.-B.; Li, S.-F.; Liu, H.-X.; Jiang, Y.-Y.; Fu, H . RSC Ad. 2012, 2, 6549. |
| [9] |
Shen, C.-R.; Spannenberg, A.; Wu, X.-F . Angew. Chem., Int. E. 2016, 55, 5067.
doi: 10.1002/anie.v55.16 |
| [10] |
(a) Pan, X.-Q.; Zou, J.-P.; Zhang, G.-L.; Zhang, W . Chem. Commu. 2010, 46, 1721.
doi: 10.1039/b925951a |
|
(b) Yan, Z.-F.; Xie, J.; Zhu, C.-J . Adv. Synth. Cata. 2017, 359, 4153.
doi: 10.1039/b925951a |
|
|
(c) Pan, C.-D.; Huang, B.-F.; Hu, W.-M.; Feng, X.-M.; Yu, J.-T . J. Org. Chem. 2016, 81, 2087.
doi: 10.1039/b925951a |
|
|
(d) Zhang, Y.; Ye, S.-Y.; Ji, M.-M.; Li, L.-S.; Guo, D.-M.; Zhu, G.-G . J. Org. Chem. 2017, 82, 6811.
doi: 10.1039/b925951a |
|
|
(e) Zhang, Y.; Guo, D.-M.; Ye, S.-Y.; Liu, Z.-C.; Zhu, G.-G . Org. Lett. 2017, 19, 1302.
doi: 10.1039/b925951a |
|
|
(f) Zhou, N.-N.; Yang, Z.-F.; Zhang, H.-L.; Wu, Z.-K.; Zhu, C.-J . J. Org. Chem. 2016, 81, 12181.
doi: 10.1039/b925951a |
|
|
(g) Zhang, Y.; Zhang, J.-H.; Hu, B.-Y.; Ji, M.-M.; Ye, S.-Y.; Zhu, G.-G . Org. Lett. 2018, 20, 2988.
doi: 10.1039/b925951a |
|
| [11] |
(a) Hari, D. P.; Hering, T.; Kcning, B . Org. Let. 2012, 14, 5334.
doi: 10.1021/ol302517n |
|
(b) Staples, M. K.; Grange, R. L.; Angus, J. A.; Ziogas, J.; Tan, N. P. H.; Taylor, K. T.; Schiesser, C. H . Org. Biomol. Che. 2011, 9, 473.
doi: 10.1021/ol302517n |
|
|
(c) Leardini, R.; Pedulli, G. F.; Tundo, A.; Huffman Jr, L. G . Synthesis. 2000, 970.
doi: 10.1021/ol302517n |
|
|
(d) Zang, H.; Sun, J. G.; Dong, X.; Li, P.; Zhang, B . Adv. Synth. Catal. 2016, 358, 1746.
doi: 10.1021/ol302517n |
|
|
(e) Yang, W.-C.; Wei, K.; Sun, X.; Zhu, J.; Wu, L . Org. Lett. 2018, 20, 3144.
doi: 10.1021/ol302517n |
|
|
(f) Xu, J.; Yu, X.-X.; Yan, J.-X.; Song, Q . Org. Lett. 2017, 19, 6292.
doi: 10.1021/ol302517n |
|
|
(g) Gao, Y.-Z.; Zhang, P.-B.; Li, G.; Zhao, Y.-F . J. Org. Chem. 2018, 83, 13726.
doi: 10.1021/ol302517n |
|
|
(h) Yan, J.-X.; Xu, J.; Zhou, Y.; Chen, J.; Song, Q . Org. Chem. Front. 2018, 5, 1483.
doi: 10.1021/ol302517n |
|
|
(i) Liu, W.; Hu, Y.-Q.; Hong, X.-Y.; Li, G.-X.; Huang, X.-B.; Gao, W.-X.; Liu, M.-C.; Xia, Y.; Zhou, Y.-B.; Wu, H.-Y . Chem. Commun. 2018, 54, 14148.
doi: 10.1021/ol302517n |
|
| [12] |
Xu, J.; Zhang, F.; Zhang, S.-F.; Zhang, L.; Yu, X.-X.; Yan, J.-X.; Song, Q . Org. Let. 2019, 21, 1112.
doi: 10.1021/acs.orglett.9b00023 |
| [13] |
Liu, Q.-Y.; Zhao, X.-H.; Li, J.-L.; Cao, S . Acta Chim. Sinic. 2018, 76, 945.
doi: 10.6023/A18080322 |
|
( 刘青雲, 赵祥虎, 李佳录, 曹松 , 化学学报 2018, 76, 945.)
doi: 10.6023/A18080322 |
| [1] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [2] | 李雅宁, 王晓艳, 唐勇. 自由基聚合的立体选择性调控★[J]. 化学学报, 2024, 82(2): 213-225. |
| [3] | 邓沈娜, 彭常春, 牛云宏, 许云, 张云霄, 陈祥, 王红敏, 刘珊珊, 沈晓. 自由基Brook重排调控的α-氟烷基-α-硅基甲醇参与的烯烃双官能团化反应[J]. 化学学报, 2024, 82(2): 119-125. |
| [4] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [5] | 李珊, 路俊欣, 刘杰, 蒋绿齐, 易文斌. 氟烷基亚磺酸钠盐电化学合成α-氟烷基酮[J]. 化学学报, 2024, 82(2): 110-114. |
| [6] | 任妍妍, 李欣, 韩英锋. 基于氮杂环卡宾蓝光有机自由基的合成及其光学性质研究★[J]. 化学学报, 2023, 81(7): 735-740. |
| [7] | 杨爽, 王宁宜, 杭青青, 张宇辰, 石枫. 邻羟基苯基取代的对亚甲基苯醌参与的催化不对称反应研究进展★[J]. 化学学报, 2023, 81(7): 793-808. |
| [8] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [9] | 黄家翩, 刘飞, 吴劼. 二氟环丙烯参与的有机反应研究进展★[J]. 化学学报, 2023, 81(5): 520-532. |
| [10] | 杨洁, 凌琳, 李玉学, 吕龙. 高氯酸铵热分解机理的密度泛函理论研究[J]. 化学学报, 2023, 81(4): 328-337. |
| [11] | 赵亚婷, 刘帆, 汪秋安, 夏吾炯. 可见光促进(氮杂)芳香胺与重氮乙酸乙酯的N-烷基化反应[J]. 化学学报, 2023, 81(2): 111-115. |
| [12] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [13] | 张红丹, 兰欣雨, 程鹏. 羟基自由基辅助沸石分子筛合成的研究进展[J]. 化学学报, 2023, 81(1): 100-110. |
| [14] | 李小娟, 叶梓瑜, 谢书涵, 王永净, 王永好, 吕源财, 林春香. 氮氯共掺杂多孔碳活化过一硫酸盐降解苯酚的性能及机理研究[J]. 化学学报, 2022, 80(9): 1238-1249. |
| [15] | 岳广禄, 魏婧瑶, 邱頔, 莫凡洋. 芳基锡烷的合成研究进展[J]. 化学学报, 2022, 80(7): 956-969. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||