化学学报 ›› 2021, Vol. 79 ›› Issue (5): 678-684.DOI: 10.6023/A21010019 上一篇
研究论文
李童心1, 李东林1,*( ), 张清波1, 高建行1, 孔祥泽1, 樊小勇1, 苟蕾1
), 张清波1, 高建行1, 孔祥泽1, 樊小勇1, 苟蕾1
投稿日期:2021-01-21
发布日期:2021-03-31
通讯作者:
李东林
基金资助:
Tongxin Li1, Donglin Li1,*( ), Qingbo Zhang1, Jianhang Gao1, Xiangze Kong1, Xiaoyong Fan1, Lei Gou1
), Qingbo Zhang1, Jianhang Gao1, Xiangze Kong1, Xiaoyong Fan1, Lei Gou1
Received:2021-01-21
Published:2021-03-31
Contact:
Donglin Li
About author:Supported by:文章分享

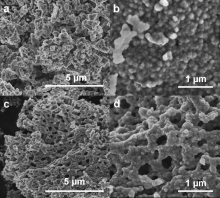
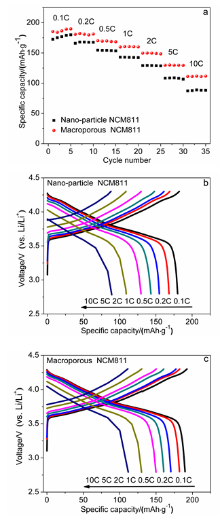
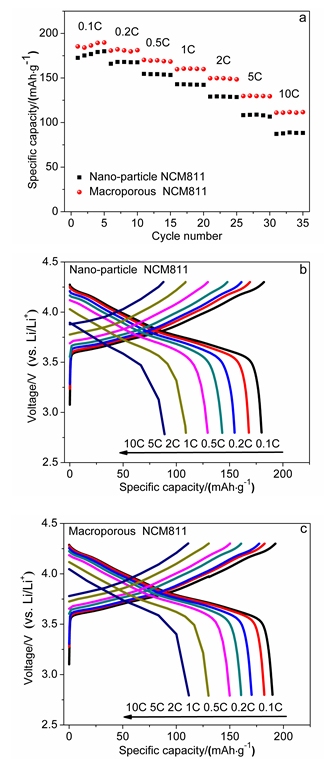
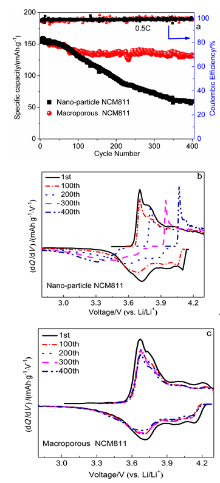
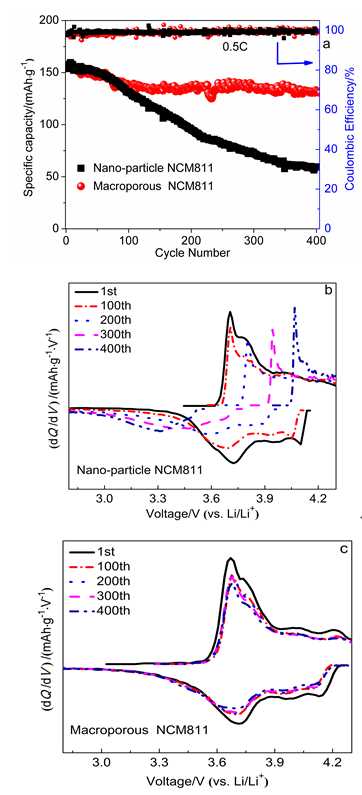
本工作以聚甲基丙烯酸甲酯(PMMA)微球组装成的胶晶模板作为铸模, 溶胶-凝胶法辅助获得大孔LiNi0.8Co0.1Mn0.1O2 (NCM811)正极材料. 结果表明, 利用PMMA作为造孔剂, 形成了由100 nm的颗粒堆积而成的大孔结构, 这种结构有效地提高了材料的倍率性能和循环稳定性. 大孔NCM811在0.1C的首次放电比容量为190.3 mAh∙g-1. 2C倍率下NCM811纳米颗粒的放电比容量仅为129.3 mAh∙g-1, 而大孔NCM811的放电比容量为149.8 mAh∙g-1. 0.5C倍率下循环400次后大孔NCM811的容量保持率为83.02%, 明显高于纳米颗粒材料的38.59%.
李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684.
Tongxin Li, Donglin Li, Qingbo Zhang, Jianhang Gao, Xiangze Kong, Xiaoyong Fan, Lei Gou. Preparation and Electrochemical Performance of Macroporous Ni-rich LiNi0.8Co0.1Mn0.1O2 Cathode Material[J]. Acta Chimica Sinica, 2021, 79(5): 678-684.
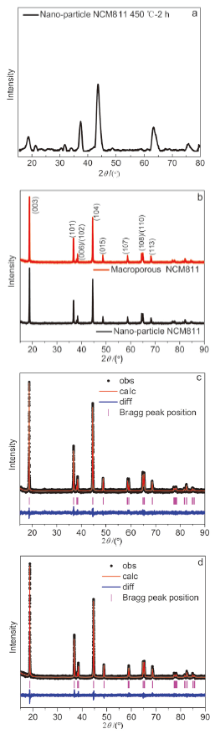
| 样品 | a/nm | c/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|
| NCM811纳米颗粒 | 0.28735 | 1.42109 | 4.9455 | 1.227 |
| 大孔NCM811 | 0.28741 | 1.42237 | 4.9489 | 1.426 |
| 样品 | a/nm | c/nm | c/a | I(003)/I(104) |
|---|---|---|---|---|
| NCM811纳米颗粒 | 0.28735 | 1.42109 | 4.9455 | 1.227 |
| 大孔NCM811 | 0.28741 | 1.42237 | 4.9489 | 1.426 |
| Parameter | NCM811纳米颗粒 | 大孔NCM811 |
|---|---|---|
| Li1(3a) | 0.9468 | 0.9611 |
| Ni1(3a) | 0.0532 | 0.0389 |
| Li2(3b) | 0.0532 | 0.0389 |
| Ni2(3b) | 0.7468 | 0.7611 |
| Co1(3b) | 0.1000 | 0.1000 |
| Mn1(3b) | 0.1000 | 0.1000 |
| O1(6c) | 1.0000 | 1.0000 |
| Ni在Li位的占比/% | 5.32 | 3.89 |
| Rp/% | 6.21 | 6.25 |
| Rwp/% | 7.94 | 7.92 |
| χ2 | 1.144 | 1.130 |
| Parameter | NCM811纳米颗粒 | 大孔NCM811 |
|---|---|---|
| Li1(3a) | 0.9468 | 0.9611 |
| Ni1(3a) | 0.0532 | 0.0389 |
| Li2(3b) | 0.0532 | 0.0389 |
| Ni2(3b) | 0.7468 | 0.7611 |
| Co1(3b) | 0.1000 | 0.1000 |
| Mn1(3b) | 0.1000 | 0.1000 |
| O1(6c) | 1.0000 | 1.0000 |
| Ni在Li位的占比/% | 5.32 | 3.89 |
| Rp/% | 6.21 | 6.25 |
| Rwp/% | 7.94 | 7.92 |
| χ2 | 1.144 | 1.130 |

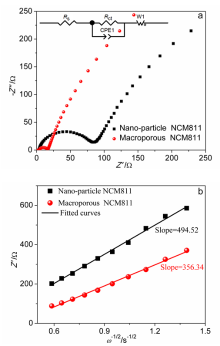
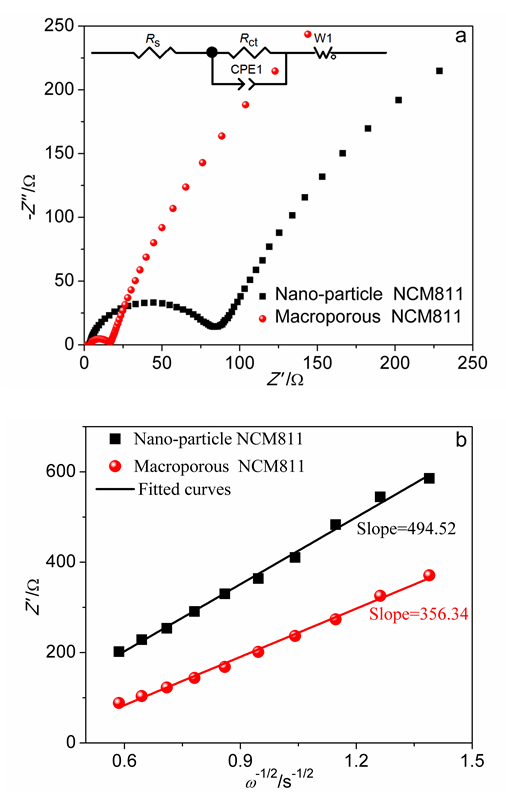
| 样品 | Cycle | Rs/Ω | Rct/Ω |
|---|---|---|---|
| NCM811纳米颗粒 | 115 | 2.246 | 66.52 |
| 大孔NCM811 | 115 | 3.621 | 10.94 |
| 样品 | Cycle | Rs/Ω | Rct/Ω |
|---|---|---|---|
| NCM811纳米颗粒 | 115 | 2.246 | 66.52 |
| 大孔NCM811 | 115 | 3.621 | 10.94 |
| [1] |
Lee, J.; Kitchaev, D.-A.; Kwon, D.-H.; Lee, C.-W.; Papp, J.-K.; Liu, Y.-S.; Lun, Z.-Y.; Clément, R.-J.; Shi, T.; McCloskey, B.-D.; Guo, J.-H.; Balasubramanian, M.; Ceder, G. Nature 2018, 556,185.
doi: 10.1038/s41586-018-0015-4 |
| [2] |
Qiao, Q.-Q.; Zhang, H.-Z.; Li, G.-R.; Ye, S.-H.; Wang, C.-W.; Gao, X.-P. J. Mater. Chem. A 2013, 1,5262.
doi: 10.1039/c3ta00028a |
| [3] |
Liu, J.-D.; Zhang, Y.-D.; Liu, J.-X.; Li, J.-H.; Qiu, X.-G.; Cheng, F.-Y. Acta Chim. Sinica 2020, 78,1426. (in Chinese).
doi: 10.6023/A20070330 |
|
( 刘九鼎, 张宇栋, 刘俊祥, 李金瀚, 邱晓光, 程方益, 化学学报, 2020, 78,1426.)
doi: 10.6023/A20070330 |
|
| [4] |
Li, Z.; Wang, Z.; Ban, L.-Q.; Wang, J.-T.; Lu, S.-G. Acta Chim. Sinica 2019, 77,1115. (in Chinese).
doi: 10.6023/A19070265 |
|
( 李钊, 王忠, 班丽卿, 王建涛, 卢世刚, 化学学报, 2019, 77,1115.)
doi: 10.6023/A19070265 |
|
| [5] |
Zheng, J.-C.; Yang, Z.; He, Z.-J.; Tong, H.; Yu, W.-J.; Zhang, J.-F. Nano Energy 2018, 53,613.
doi: 10.1016/j.nanoen.2018.09.014 |
| [6] |
Liu, Y.-Y.; Yang, Z.; Li, J.-L.; Niu, B.-B.; Yang, K.; Kang, F.-Y. J. Mater. Chem. A 2018, 6,13883.
doi: 10.1039/C8TA04568B |
| [7] |
Zhang, X.-D.; Hao, J.-J.; Wu, L.-C.; Guo, Z.-M.; Ji, Z.-H.; Luo, J.; Chen, C.-G.; Shu, J.-F.; Long, H.-M.; Yang, F.; Volinsky, A.-A. Electrochim. Acta 2018, 283,1203.
doi: 10.1016/j.electacta.2018.07.057 |
| [8] |
Li, Y.-C.; Zhao, W.-M.; Xiang, W.; Wu, Z.-G.; Yang, Z.-G.; Xu, C.-L.; Xu, Y.-D.; Wang, E.-H.; Wu, C.-J.; Guo, X.-D. J. Alloys Compd. 2018, 766,546.
doi: 10.1016/j.jallcom.2018.06.364 |
| [9] |
Xu, X.; Huo, H.; Jian, J.-Y.; Wang, L.-G.; Zhu, H.; Xu, S.; He, X.-S.; Yin, G.-P.; Du, C.-Y.; Sun, X.-L. Adv. Energy Mater. 2019, 9,1803963.
doi: 10.1002/aenm.v9.15 |
| [10] |
Zou, P.-J.; Lin, Z.-H.; Fan, M.-N.; Wang, F.; Liu, Y.; Xiong, X.-H. Appl. Surf. Sci. 2020, 504,144506.
doi: 10.1016/j.apsusc.2019.144506 |
| [11] |
Su, Y.-F.; Chen, G.; Chen, L.; Li, W.-K.; Zhang, Q.-Y.; Yang, Z.-R.; Lu, Y.; Bao, L.-Y.; Tan, J.; Chen, R.-J.; Chen, S.; Wu, F. ACS Appl. Mater. Interfaces 2018, 10,6407.
doi: 10.1021/acsami.7b18933 |
| [12] |
Chen, T.; Wang, F.; Li, X.; Yan, X.-X.; Wang, H.; Deng, B.-W.; Xie, Z.-W.; Qu, M.-Z. Appl. Surf. Sci. 2019, 465,863.
doi: 10.1016/j.apsusc.2018.09.250 |
| [13] |
Huang, B.; Wang, M.; Zhang, X.-W.; Zhao, Z.-Y.; Chen, L.; Gu, Y.-J. J. Alloys Compd. 2020, 830,154619.
doi: 10.1016/j.jallcom.2020.154619 |
| [14] |
Yang, H.-P.; Wu, H.-H.; Ge, M.-Y.; Li, L.-J.; Yuan, Y.-F.; Yao, Q.; Chen, J.; Xia, L.-F.; Zheng, J.-M.; Chen, Z.-Y.; Duan, J.-F.; Kisslinger, K.; Zeng, X.-C.; Lee, W.-K.; Zhang, Q.-B.; Lu, J. Adv. Funct. Mater. 2019, 29,1808825.
doi: 10.1002/adfm.v29.13 |
| [15] |
Zhu, H.-W.; Yu, H.-F.; Jiang, H.-B.; Hu, Y.-J.; Jiang, H.; Li, C.-Z. Chem. Eng. Sci. 2020, 217,115518.
doi: 10.1016/j.ces.2020.115518 |
| [16] |
Ryu, H.-H.; Park, K.-J.; Yoon, D.-R.; Aishova, A.; Yoon, C.-S.; Sun, Y.-K. Adv. Energy Mater. 2019, 9,1902698.
doi: 10.1002/aenm.v9.44 |
| [17] |
Song, B.-H.; Li, W.-D.; Oh, S.-M.; Manthiram, A. ACS Appl. Mater. Interfaces 2017, 9,9718.
doi: 10.1021/acsami.7b00070 |
| [18] |
Wang, M.; Zhang, R.; Gong, Y.-Q.; Su, Y.-F.; Xiang, D.-B.; Chen, L.; Chen, Y.-B.; Luo, M.; Chu, M. Solid State Ionics 2017, 312,53.
doi: 10.1016/j.ssi.2017.10.017 |
| [19] |
Kong, J.-Z.; Zhai, H.-F.; Ren, C.; Gao, M.-Y.; Zhang, X.; Li, H.; Li, J.-X.; Tang, Z.; Zhou, F. J. Alloys Compd. 2013, 577,507.
doi: 10.1016/j.jallcom.2013.07.007 |
| [20] |
Li, D.-L.; Tian, M.; Xie, R.; Li, Q.; Fan, X.-Y.; Gou, L.; Zhao, P.; Ma, S.-L.; Shi, Y.-X.; Yong, H.-T.-H. Nanoscale 2014, 6,3302.
doi: 10.1039/c3nr04927b |
| [21] |
Li, S.; Ma, G.; Guo, B.; Yang, Z.-H.; Fan, X.-M.; Chen, Z.-X.; Zhang, W.-X. Ind. Eng. Chem. Res. 2016, 55,9352.
doi: 10.1021/acs.iecr.6b02463 |
| [22] |
Li, L.-J.; Xu, M.; Yao, Q.; Chen, Z.-Y.; Song, L.-B.; Zhang, Z.-A.; Gao, C.-H.; Wang, P.; Yu, Z.-Y.; Lai, Y.-Q. ACS Appl. Mater. Interfaces 2016, 8,30879.
doi: 10.1021/acsami.6b09197 |
| [23] |
Ren, D.; Yang, Y.; Shen, L.-X.; Zeng, R.; Abruña, H.-D. J. Power Sources 2020, 447,227344.
doi: 10.1016/j.jpowsour.2019.227344 |
| [24] |
Su, Y.-F.; Zhang, Q.-Y.; Chen, L.; Bao, L.-Y.; Lu, Y.; Chen, S.; Wu, F. Acta Phys.-Chim. Sin. 2021, 37,1. (in Chinese).
|
|
( 苏岳锋, 张其雨, 陈来, 包丽颖, 卢赟, 陈实, 吴锋, 物理化学学报, 2021, 37,1.)
|
|
| [25] |
Gao, S.; Cheng, Y.-T.; Shirpour, M. ACS Appl. Mater. Interfaces 2019, 11,982.
doi: 10.1021/acsami.8b19349 |
| [26] |
Liu, W.; Li, X.-F.; Xiong, D.-B.; Hao, Y.-C.; Li, J.-W.; Kou, H.-R.; Yan, B.; Li, D.-J.; Lu, S.-G.; Koo, A.; Adair, K.; Sun, X.-L. Nano Energy 2018, 44,111.
doi: 10.1016/j.nanoen.2017.11.010 |
| [27] |
Tang, Z.-H.; Zheng, H.-H.; Qian, F.-P.; Ma, Y.-H.; Zhao, C.-Y.; Song, L.-B.; Chen, Y.; Xiong, X.; Zhu, X.-X.; Mi, C. Ionics 2018, 24,61.
doi: 10.1007/s11581-017-2179-6 |
| [28] |
Luo, D.; Li, G.-S.; Fu, C.-C.; Zheng, J.; Fan, J.-M.; Li, Q.; Li, L.-P. Adv. Energy Mater. 2014, 4,1400062.
doi: 10.1002/aenm.201400062 |
| [29] |
Wang, H.; Ge, W.-J.; Li, W.; Wang, F.; Liu, W.-J.; Qu, M.-Z.; Peng, G.-C. ACS Appl. Mater. Interfaces 2016, 8,18439.
doi: 10.1021/acsami.6b04644 |
| [30] |
Aishova, A.; Park, G.-T.; Yoon, C.-S.; Sun, Y.-K. Adv. Energy Mater. 2020, 10,1903179.
doi: 10.1002/aenm.v10.4 |
| [31] |
Song, X.; Liu, G.-X.; Yue, H.-F.; Luo, L.; Yang, S.-Y.; Huang, Y.-Y.; Wang, C.-R. Chem. Eng. J. 2021, 407,126301.
doi: 10.1016/j.cej.2020.126301 |
| [32] |
Zheng, Z.; Wu, Z.-G.; Xiang, W.; Guo, X.-D. Acta Chim. Sinica 2017, 75,501. (in Chinese).
doi: 10.6023/A16110594 |
|
( 郑卓, 吴振国, 向伟, 郭孝东, 化学学报, 2017, 75,501.)
doi: 10.6023/A16110594 |
|
| [33] |
Zhu, X.-J.; Chen, H.-H.; Zhan, H.; Liu, H.-X.; Yang, D.-L.; Zhou, Y.-H. Chin. J. Chem. 2005, 23,491.
doi: 10.1002/(ISSN)1614-7065 |
| [34] |
Song, J.-W.; Kim, J.-Y.; Kang, T.-W.; Kim, D.-C. Sci. Rep. 2017, 7,42521.
doi: 10.1038/srep42521 |
| [35] |
Ho, V.-C.; Jeong, S.-H.; Yim, T.; Mun, J.-Y. J. Power Sources 2020, 450,227625.
doi: 10.1016/j.jpowsour.2019.227625 |
| [36] |
Ding, G.-Y.; Gao, Y.; Li, Y.-H.; Zhu, Z.; Wang, Q.-L.; Jing, X.-G.; Yan, F.-Q.; Xu, G.-J.; Yue, Z.-H.; Li, X.-M.; Sun, F.-G. Chin. J. Inorg. Chem. 2020, 36,2307. (in Chinese).
|
|
( 丁国彧, 高远, 李亚辉, 朱振, 王秋琳, 景鑫国, 严奉乾, 徐国军, 岳之浩, 李晓敏, 孙福根, 无机化学学报, 2020, 36,2307.)
|
|
| [37] |
He, Y.-L.; Li, Y.; Liu, Y.; Li, W.-X.; Liu, W.-B. Mater. Chem. Phys. 2020, 251,123085.
doi: 10.1016/j.matchemphys.2020.123085 |
| [38] |
Yao, W.-L.; Liu, Y.; Li, D.; Zhang, Q.; Zhong, S.-W.; Cheng, H.-W.; Yan, Z.-Q. J. Phys. Chem. C 2020, 124,2346.
doi: 10.1021/acs.jpcc.9b10526 |
| [39] |
Sim, S.-J.; Lee, S.-H.; Jin, B.-S.; Kim, H.-S. Sci. Rep. 2019, 9,8952.
doi: 10.1038/s41598-019-45556-7 |
| [40] |
Wu, Z.-Z.; Ji, S.-P.; Liu, T.-C.; Duan, Y.-D.; Xiao, S.; Lin, Y.; Xu, K.; Pan, F. Nano Lett. 2016, 16,6357.
doi: 10.1021/acs.nanolett.6b02742 |
| [41] |
Zhu, W.; Tai, Z.-G.; Shu, C.-Y.; Chong, S.-K.; Guo, S.-W.; Ji, L.-J.; Chen, Y.-Z.; Liu, Y.-N. J. Mater. Chem. A 2020, 8,7991.
doi: 10.1039/D0TA00355G |
| [42] |
Li, L.-J.; Chen, Z.-Y.; Zhang, Q.-B.; Xu, M.; Zhou, X.; Zhu, H.-L.; Zhang, K.-L. J. Mater. Chem. A 2015, 3,894.
doi: 10.1039/C4TA05902F |
| [1] | 顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151. |
| [2] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [3] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [4] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [5] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [6] | 邓权政, 毛文婷, 韩璐. 介观尺度多孔材料的电子显微学结构解析[J]. 化学学报, 2022, 80(8): 1203-1216. |
| [7] | 何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征. 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022, 80(7): 896-902. |
| [8] | 陈守潇, 刘君珂, 郑伟琛, 魏国祯, 周尧, 李君涛. 电/离子导体双包覆的LiNi0.8Co0.1Mn0.1O2锂离子电池阴极材料及其电化学性能[J]. 化学学报, 2022, 80(4): 485-493. |
| [9] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [10] | 薛晓兰, 张洋, 石美瑜, 李天琳, 黄天龙, 戚继球, 委福祥, 隋艳伟, 金钟. 有机电极材料在非水系金属镁二次电池中的研究进展[J]. 化学学报, 2022, 80(12): 1618-1628. |
| [11] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [12] | 徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081. |
| [13] | 穆春辉, 张艺馨, 寇伟, 徐联宾. 镍氮掺杂有序大孔/介孔碳负载银纳米颗粒用于高效电催化CO2还原[J]. 化学学报, 2021, 79(7): 925-931. |
| [14] | 林碧霞, 黄颖珊, 陈帅, 邢震宇. 钠硒电池关键材料的研究进展[J]. 化学学报, 2021, 79(5): 641-648. |
| [15] | 张璐, 王文凤, 张洪明, 韩树民, 王利民. 水系锌离子电池研究进展和挑战[J]. 化学学报, 2021, 79(2): 158-175. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||