化学学报 ›› 2022, Vol. 80 ›› Issue (12): 1618-1628.DOI: 10.6023/A22090385 上一篇 下一篇
综述
薛晓兰a, 张洋a, 石美瑜a, 李天琳a, 黄天龙a, 戚继球a, 委福祥a, 隋艳伟a,*( ), 金钟b,*(
), 金钟b,*( )
)
投稿日期:2022-09-05
发布日期:2022-10-27
通讯作者:
隋艳伟, 金钟
作者简介: |
薛晓兰, 2015年于陕西师范大学化学化工学院获学士学位, 2020年于南京大学化学化工学院获博士学位. 2020年被聘为中国矿业大学材料与物理学院讲师. 主要研究方向为新型多价态金属离子电池电极材料的设计合成及储能机制研究. |
 |
隋艳伟, 2003年于哈尔滨理工大学获学士学位, 2009年于哈尔滨工业大学获博士学位. 2009年被聘为中国矿业大学材料与物理学院讲师, 2016年于新西兰奥克兰大学进行访学, 2020年被聘为中国矿业大学材料与物理学院教授. 主要从事新能源材料与器件和合金材料及功能化方面的研究. |
 |
金钟, 2003年于北京大学化学与分子工程学院获学士学位, 2008年于北京大学化学与分子工程学院获博士学位. 2008年和2010年分别于美国莱斯大学和麻省理工学院进行博士后研究. 2014年被聘为南京大学化学化工学院教授. 主要从事清洁能源转换/存储材料及器件和新型纳米材料性质调控与光电功能器件方面的研究. |
基金资助:
Xiaolan Xuea, Yang Zhanga, Meiyu Shia, Tianlin Lia, Tianlong Huanga, Jiqiu Qia, Fuxiang Weia, Yanwei Suia( ), Zhong Jinb(
), Zhong Jinb( )
)
Received:2022-09-05
Published:2022-10-27
Contact:
Yanwei Sui, Zhong Jin
Supported by:文章分享

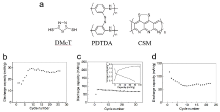
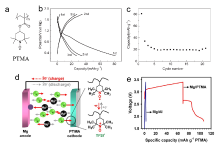
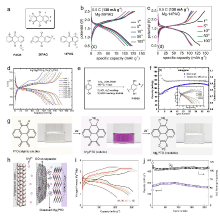
由于镁资源储量丰富、成本低廉, 且金属镁具有理论体积比容量高(3833 mAh/cm3), 沉积/溶解过程中不易形成枝晶等优点, 金属镁二次电池受到了研究者的广泛关注. 然而, Mg2+较大的极性导致其在多数锂离子电池正极材料中无法实现可逆脱嵌. 主流无机电极材料普遍存在只能在较小电流密度下循环、动力学缓慢、制备工艺复杂等问题. 相较而言, 有机电极材料具有理论比容量高、结构多样易调控、资源丰富、环境友好、受离子半径和电荷影响小等优点, 被认为是一种有潜力的电极材料. 综述了近年来用于非水系镁二次电池有机正极材料的研究进展, 讨论了不同类型有机正极材料的电荷存储机制及电化学性能, 并总结了其面临的挑战、解决策略以及未来的发展方向.
薛晓兰, 张洋, 石美瑜, 李天琳, 黄天龙, 戚继球, 委福祥, 隋艳伟, 金钟. 有机电极材料在非水系金属镁二次电池中的研究进展[J]. 化学学报, 2022, 80(12): 1618-1628.
Xiaolan Xue, Yang Zhang, Meiyu Shi, Tianlin Li, Tianlong Huang, Jiqiu Qi, Fuxiang Wei, Yanwei Sui, Zhong Jin. Recent Progress on Organic Electrode Materials for Nonaqueous Magnesium Secondary Batteries[J]. Acta Chimica Sinica, 2022, 80(12): 1618-1628.








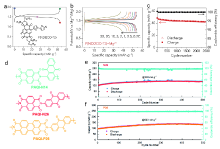
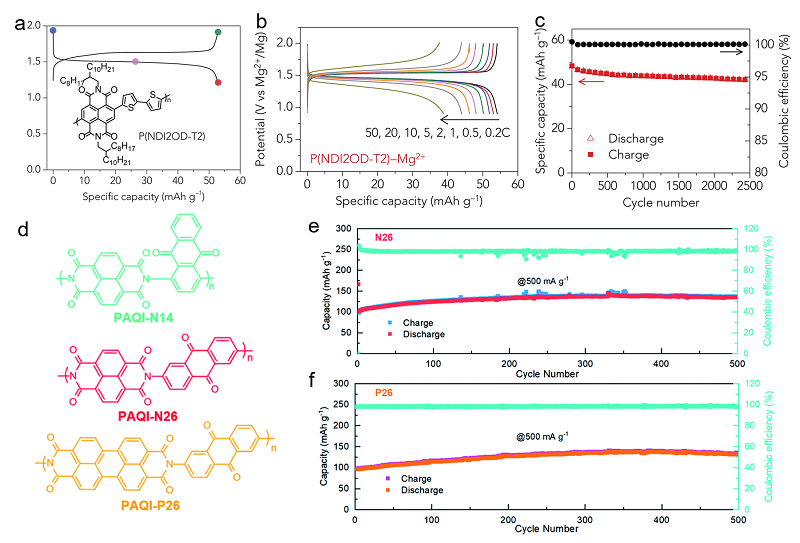

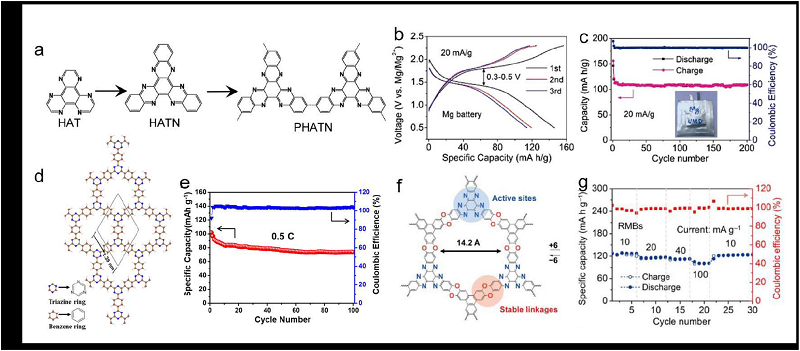
| [1] |
Dunn, B.; Kamath, H.; Tarascon, J. M. Science 2011, 334, 928.
|
| [2] |
Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Energy Environ. Sci. 2011, 4, 3243.
|
| [3] |
Melot, B. C.; Tarascon, J. M. Acc. Chem. Res. 2013, 46, 1226.
|
| [4] |
Lei, X.; Liang, X.; Yang, R.; Zhang, F.; Wang, C.; Lee, C. S.; Tang, Y. Small 2022, 18, 2200418.
|
| [5] |
Yoo, H. D.; Shterenberg, I.; Gofer, Y.; Gershinsky, G.; Pour, N.; Aurbach, D. Energy Environ. Sci. 2013, 6, 2265.
|
| [6] |
Gregory, T. D.; Hoffman, R. J.; Winterton, R. C. J. Electrochem. Soc. 1990, 137, 775.
|
| [7] |
Aurbach, D.; Lu, Z.; Schechter, A.; Gofer, Y.; Gizbar, H.; Turgeman, R.; Cohen, M.; Moshkovich, M.; Levi, E. Nature 2000, 407, 724.
|
| [8] |
Zhang, R.; Yu, X.; Nam, K. W.; Ling, C.; Arthur, T. S.; Song, W.; Knapp, A. M.; Ehrlich, S. N.; Yang, X.; Matsui, M. Electrochem. Commun. 2012, 23, 110.
|
| [9] |
Cheng, Y.; Shao, Y.; Raju, V.; Ji, X.; Mehdi, B. L.; Han, K. S.; Engelhard, M. H.; Li, G.; Browning, N. D.; Mueller, K. T.; Liu, J. Adv. Funct. Mater. 2016, 26, 3446.
|
| [10] |
Wang, L.; Asheim, K.; Vullum, P. E.; Svensson, A. M.; Vullum- Bruer, F. Chem. Mater. 2016, 28, 6459.
|
| [11] |
Truong, Q. D.; Devaraju, M. K.; Honma, I. J. Power Sources 2017, 361, 195.
|
| [12] |
NuLi, Y.; Yang, J.; Li, Y.; Wang, J. Chem. Commun. 2010, 46, 3794.
|
| [13] |
Sun, X.; Bonnick, P.; Nazar, L. F. ACS Energy Lett. 2 016, 1, 297.
|
| [14] |
Liang, Y.; Feng, R.; Yang, S.; Ma, H.; Liang, J.; Chen, J. Adv. Mater. 2012, 23, 640.
|
| [15] |
Liu, B.; Luo, T.; Mu, G.; Wang, X.; Chen, D.; Shen, G. ACS Nano 2013, 7, 8051.
|
| [16] |
Duffort, V.; Sun, X.; Nazar, L. F. Chem. Commun. 2016, 52, 12458.
|
| [17] |
Ding, Y.; Ren, X.; Chen, D.; Wen, F.; Li, T.; Xu, F. ACS Appl. Energy Mater. 2022, 5, 3004.
|
| [18] |
Sun, X.; Bonnick, P.; Duffort, V.; Liu, M.; Rong, Z.; Persson, K. A.; Ceder, G.; Nazar, L. F. Energy Environ. Sci. 2016, 9, 2273.
|
| [19] |
Tang, M.; Li, H.; Wang, E.; Wang, C. Chin. Chem. Lett. 2018, 29, 232.
|
| [20] |
Dawut, G.; Lu, Y.; Zhao, Q.; Liang, J.; Tao, Z. L.; Chen, J. Acta Phys.-Chim. Sin. 2016, 32, 1593. (in Chinese)
|
|
( 古丽巴哈尔•达吾提, 卢勇, 赵庆, 梁静, 陶占良, 陈军, 物理化学学报, 2016, 32, 1593.)
|
|
| [21] |
McAllister, B. T.; Kyne, L. T.; Schon, T. B.; Seferos, D. S. Joule 2019, 3, 620.
doi: 10.1016/j.joule.2018.12.005 |
| [22] |
Qin, K.; Huang, J.; Holguin, K.; Luo, C. Energy Environ. Sci. 2020, 13, 3950.
|
| [23] |
Song, Z.; Zhou, H. Energy Environ. Sci. 2013, 6, 2280.
|
| [24] |
Williams, D. L.; Byrne, J. J.; Driscoll, J. S. J. Electrochem. Soc. 1969, 116, 2.
|
| [25] |
Kumar, G.; Sivashanmugam, A.; Muniyandi, N.; Dhawan, S. K.; Trivedi, D. C. Synth. Met. 1996, 80, 279.
|
| [26] |
Shadike, Z.; Tan, S.; Wang, Q. C.; Lin, R.; Hu, E.; Qu, D.; Yang, X. Q. Mater. Horiz. 2021, 8, 471.
|
| [27] |
NuLi, Y.; Guo, Z.; Liu, H.; Yang, J. Electrochem. Commun. 2007, 9, 1913.
|
| [28] |
NuLi, Y.; Chen, Q.; Wang, W.; Wang, Y.; Yang, J.; Wang, J. Sci. World J. 2014, 2014, 107918.
|
| [29] |
Lu, Y.; Zhang, Q.; Li, L.; Niu, Z.; Chen, J. Chem 2018, 4, 2786.
|
| [30] |
Chen, Q.; NuLi, Y.; Guo, W.; Yang, J.; Wang, J.; Guo, Y. Acta Phys.- Chim. Sin. 2013, 29, 2295.
|
| [31] |
Ha, S. Y.; Lee, Y. W.; Woo, S. W.; Koo, B.; Kim, J. S.; Cho, J.; Lee, K.; Choi, N. S. ACS Appl. Mater. Interfaces 2014, 6, 4063.
|
| [32] |
Häupler, B.; Wild, A.; Schubert, U. S. Adv. Energy Mater. 2015, 5, 1402034.
|
| [33] |
Xie, J.; Zhang, Q. Small 2019, 15, 1805061.
|
| [34] |
Sano, H.; Senoh, H.; Yao, M.; Sakaebe, H.; Kiyobayashi, T. Chem. Lett. 2012, 41, 1594.
|
| [35] |
Senoh, H.; Sakaebe, H.; Sano, H.; Yao, M.; Kuratani, K.; Takeichi, N.; Kiyobayashi, T. J. Electrochem. Soc. 2014, 161, A1315.
|
| [36] |
Pan, B.; Zhou, D.; Huang, J.; Zhang, L.; Burrell, A. K.; Vaughey, J. T.; Zhang, Z.; Liao, C. J. Electrochem. Soc. 2016, 163, A580.
|
| [37] |
Debashis, T.; Viswanatha, H. M.; Harish, M. N. K.; Sampath, S. J. Electrochem. Soc. 2020, 167, 070561.
|
| [38] |
Bitenc, J.; Pirnat, K.; Bančič, T.; Gaberšček, M.; Genorio, B.; Randon-Vitanova, A.; Dominko, R. ChemSusChem 2015, 8, 4128.
doi: 10.1002/cssc.201500910 pmid: 26610185 |
| [39] |
Song, Z.; Qian, Y.; Gordin, M. L.; Tang, D.; Xu, T.; Otani, M.; Zhan, H.; Zhou, H. Angew. Chem. Int. Ed. 2015, 54, 13947.
|
| [40] |
Pan, B.; Huang, J.; Feng, Z.; Zeng, L.; He, M.; Zhang, L.; Vaughey, J. T.; Bedzyk, M. J.; Fenter, P.; Zhang, Z.; Burrell, A. K.; Liao, C. Adv. Energy Mater. 2016, 6, 1600140.
|
| [41] |
Bitenc, J.; Pirnat, K.; Žagar, E.; Randon-Vitanova, A.; Dominko, R. J. Power Sources 2019, 430, 90.
doi: 10.1016/j.jpowsour.2019.04.114 |
| [42] |
Bitenc, J.; Pirnat, K.; Mali, G.; Novosel, B.; Vitanova, A. R.; Dominko, R. Electrochem. Commun. 2016, 69, 1.
|
| [43] |
Tian, J.; Cao, D.; Zhou, X.; Hu, J.; Huang, M.; Li, C. ACS Nano 2018, 12, 3424.
|
| [44] |
Dong, H.; Tutusaus, O.; Liang, Y.; Zhang, Y.; Lebens-Higgins, Z.; Yang, W.; Mohtadi, R.; Yao, Y. Nat. Energy 2020, 5, 1043.
|
| [45] |
Rodríguez-Pérez, I. A.; Yuan, Y.; Bommier, C.; Wang, X.; Ma, L.; Leonard, D. P.; Lerner, M. M.; Carter, R. G.; Wu, T.; Greaney, P. A.; Lu, J.; Ji, X. J. Am. Chem. Soc. 2017, 139, 13031.
doi: 10.1021/jacs.7b06313 pmid: 28823162 |
| [46] |
Cui, L.; Zhou, L.; Zhang, K.; Xiong, F.; Tan, S.; Li, M.; An, Q.; Kang, Y. M.; Mai, L. Nano Energy 2019, 65, 103902.
|
| [47] |
Yang, H.; Xu, F. ChemPhysChem 2021, 22, 1455.
|
| [48] |
Bančič, T.; Bitenc, J.; Pirnat, K.; Lautar, A. K.; Grdadolnik, J.; Vitanova, A. R.; Dominko, R. J. Power Sources 2018, 395, 25.
|
| [49] |
Fan, X.; Wang, F.; Ji, X.; Wang, R.; Gao, T.; Hou, S.; Chen, J.; Deng, T.; Li, X.; Chen, L.; Luo, C.; Wang, L.; Wang, C. Angew. Chem. Int. Ed. 2018, 57, 7146.
|
| [50] |
Wang, Y.; Liu, Z.; Wang, C.; Hu, Y.; Lin, H.; Kong, W.; Ma, J.; Jin, Z. Energy Storage Mater. 2020, 26, 494.
|
| [51] |
Dong, H.; Liang, Y.; Tutusaus, O.; Mohtadi, R.; Zhang, Y.; Hao, F.; Yao, Y. Joule 2019, 3, 782.
doi: 10.1016/j.joule.2018.11.022 |
| [52] |
Ding, Y.; Chen, D.; Ren, X.; Cao, Y.; Xu, F. J. Mater. Chem. A 2022, 10, 14111.
|
| [53] |
Cao, Y.; Wang, M.; Wang, H.; Han, C.; Pan, F.; Sun, J. Adv. Energy Mater. 2022, 12, 2200057.
|
| [54] |
Li, J.; Jing, X.; Li, Q.; Li, S.; Gao, X.; Feng, X.; Wang, B. Chem. Soc. Rev. 2020, 49, 3565.
|
| [55] |
Mao, M.; Luo, C.; Pollard, T. P.; Hou, S.; Gao, T.; Fan, X.; Cui, C.; Yue, J.; Tong, Y.; Yang, G.; Deng, T.; Zhang, M.; Ma, J.; Suo, L.; Borodin, O.; Wang, C. Angew. Chem., Int. Ed. 2019, 58, 17820.
|
| [56] |
Sun, R.; Hou, S.; Luo, C.; Ji, X.; Wang, L.; Mai, L.; Wang, C. Nano Lett. 2020, 20, 3880.
|
| [57] |
Li, S.; Liu, Y.; Dai, L.; Li, S.; Wang, B.; Xie, J.; Li, P. Energy Storage Mater. 2022, 48, 439.
|
| [1] | 何家伟, 焦柳, 程雪怡, 陈光海, 吴强, 王喜章, 杨立军, 胡征. 金属有机框架衍生的空心碳纳米笼的结构调控与锂硫电池性能研究[J]. 化学学报, 2022, 80(7): 896-902. |
| [2] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [3] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [4] | 林碧霞, 黄颖珊, 陈帅, 邢震宇. 钠硒电池关键材料的研究进展[J]. 化学学报, 2021, 79(5): 641-648. |
| [5] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [6] | 李燕丽, 于丹丹, 林森, 孙东飞, 雷自强. α-MnO2纳米棒/多孔碳正极材料的制备及水系锌离子电池性能研究[J]. 化学学报, 2021, 79(2): 200-207. |
| [7] | 张璐, 王文凤, 张洪明, 韩树民, 王利民. 水系锌离子电池研究进展和挑战[J]. 化学学报, 2021, 79(2): 158-175. |
| [8] | 梁其梅, 郭昱娇, 郭俊明, 向明武, 刘晓芳, 白玮, 宁平. 亚微米去顶角八面体LiNi0.08Mn1.92O4正极材料制备及高温电化学性能[J]. 化学学报, 2021, 79(12): 1526-1533. |
| [9] | 谢佶晟, 肖竹梅, 左文华, 杨勇. 钠离子电池钴酸钠正极材料研究进展[J]. 化学学报, 2021, 79(10): 1232-1243. |
| [10] | 汤功奥, 毛鲲, 张静, 吕品, 程雪怡, 吴强, 杨立军, 王喜章, 胡征. 分级结构氮掺杂碳纳米笼:高倍率长寿命可充电镁电池正极材料[J]. 化学学报, 2020, 78(5): 444-450. |
| [11] | 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益. 磷酸锂原位包覆富锂锰基锂离子电池正极材料[J]. 化学学报, 2020, 78(12): 1426-1433. |
| [12] | 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心. 铝掺杂及钨酸锂表面包覆双效提升富锂锰基正极材料的循环稳定性[J]. 化学学报, 2020, 78(11): 1268-1274. |
| [13] | 宋学霞, 李继成, 李朝晖, 李喜飞, 丁燕怀, 肖启振, 雷钢铁. 钾掺杂对钒酸钠纳米片储钠性能的影响[J]. 化学学报, 2019, 77(7): 625-633. |
| [14] | 李钊, 王忠, 班丽卿, 王建涛, 卢世刚. 富锂锰基正极材料的表面改性研究进展[J]. 化学学报, 2019, 77(11): 1115-1128. |
| [15] | 王玲, 杨国锐, 王嘉楠, 王思岚, 彭生杰, 延卫. 静电纺丝在钠离子电池中的应用研究进展[J]. 化学学报, 2018, 76(9): 666-680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||