化学学报 ›› 2021, Vol. 79 ›› Issue (7): 932-940.DOI: 10.6023/A21030118 上一篇 下一篇
研究论文
王瑞兆a,b, 邹云杰a,b, 洪晟a,b, 徐铭楷a,b, 凌岚a,b,*( )
)
投稿日期:2021-03-29
发布日期:2021-05-31
通讯作者:
凌岚
基金资助:
Ruizhao Wanga,b, Yunjie Zoua,b, Sheng Honga,b, Mingkai Xua,b, Lan Linga,b( )
)
Received:2021-03-29
Published:2021-05-31
Contact:
Lan Ling
Supported by:文章分享

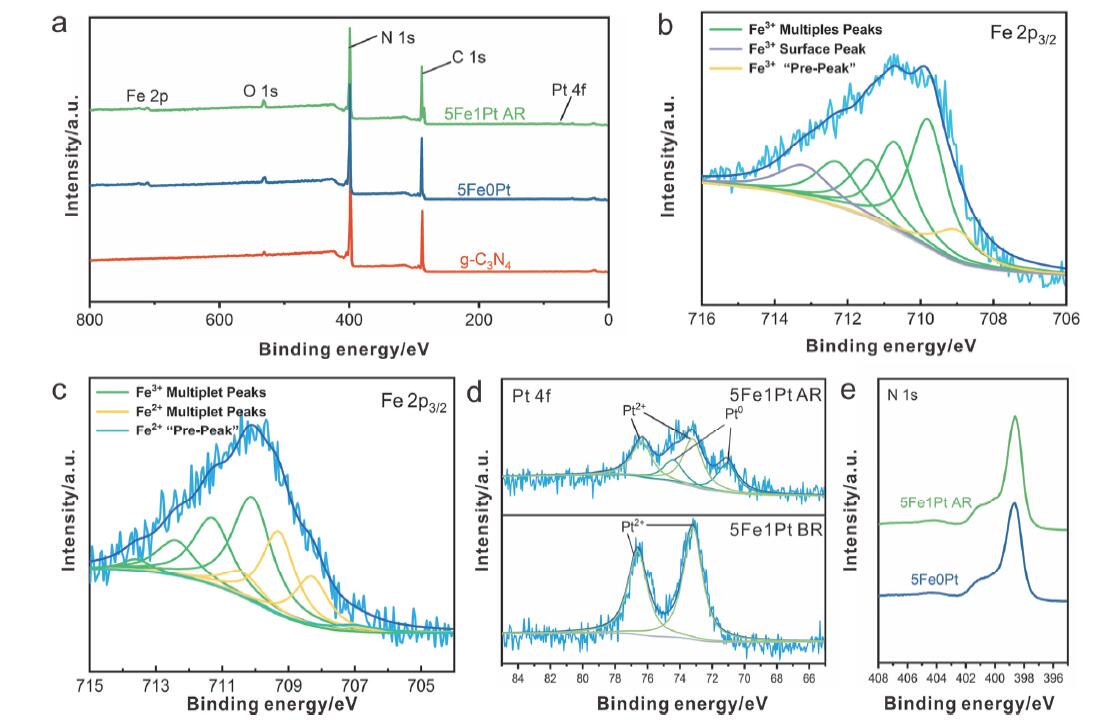
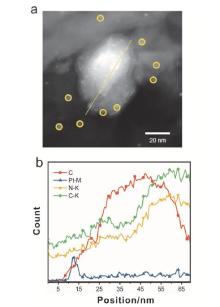
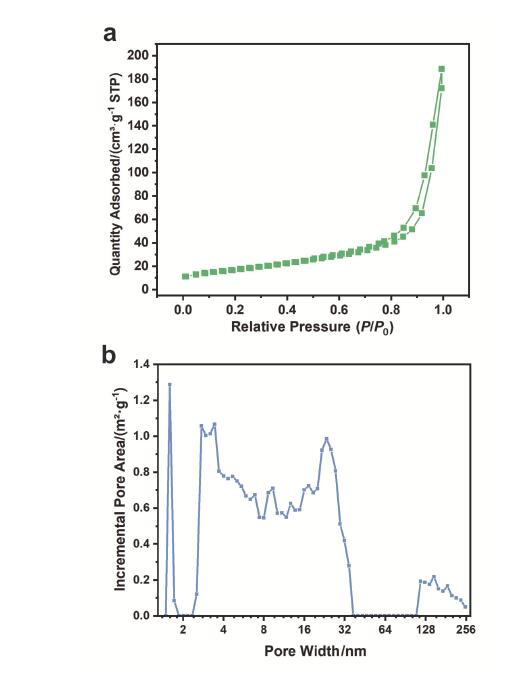
光热催化是一种高效利用太阳光, 将二氧化碳转化为高价值产物的方法. 本工作以石墨相氮化碳为载体, 通过水热-浸渍两步法制备了负载铂、铁氧化物的石墨相氮化碳催化剂. 该催化剂具备优异的光热转换性能, 可实现7.36 mmol•h-1•gcat-1的二氧化碳还原活性和97%的一氧化碳选择性. 使用X射线晶体衍射(XRD)、配备能量色散X射线谱(EDS)的球差校正扫描透射电子显微镜(Cs-S/TEM)、X射线光电子能谱(XPS)、紫外可见漫反射光谱(DRS)等表征手段从催化剂物相、微观结构、表面状态、光学性能等方面对催化剂进行了表征. 结果显示, 催化剂能吸收全谱太阳光, 且具备较高的载流子分离效率. 基于原位傅里叶变换红外光谱(DRIFTS)表征结果, 提出了二氧化碳在催化剂表面的可能的反应机理, 并对铂在铁氧化物表面的氢溢流效应进行了表征. 结果表明二氧化碳和氢气分别在铁氧化物、铂位点被活化, 参与催化反应. 本工作对后续光热二氧化碳还原催化剂的设计、合成与机理研究具有一定的参考作用.
王瑞兆, 邹云杰, 洪晟, 徐铭楷, 凌岚. Pt0.01Fe0.05-g-C3N4催化剂高效光热催化二氧化碳还原[J]. 化学学报, 2021, 79(7): 932-940.
Ruizhao Wang, Yunjie Zou, Sheng Hong, Mingkai Xu, Lan Ling. High-performance Pt0.01Fe0.05-g-C3N4 Catalyst for Photothermal Catalytic CO2 Reduction[J]. Acta Chimica Sinica, 2021, 79(7): 932-940.







| [1] |
Gao, W.; Liang, S.; Wang, R.; Jiang, Q.; Zhang, Y.; Zheng, Q.; Xie, B.; Toe, C. Y.; Zhu, X.; Wang, J.; Huang, L.; Gao, Y.; Wang, Z.; Jo, C.; Wang, Q.; Wang, L.; Liu, Y.; Louis, B.; Scott, J.; Roger, A.-C.; Amal, R.; He, H.; Park, S.-E. Chem. Soc. Rev. 2020, 49, 8584.
doi: 10.1039/D0CS00025F |
| [2] |
Ghoussoub, M.; Xia, M.; Duchesne, P. N.; Segal, D.; Ozin, G. Energy Environ. Sci. 2019, 12, 1122.
doi: 10.1039/C8EE02790K |
| [3] |
Yin, S.; Sun, L.; Zhou, Y.; Li, X.; Li, J.; Song, X.; Huo, P.; Wang, H.; Yan, Y. Chem. Eng. J. 2021, 406, 126776.
doi: 10.1016/j.cej.2020.126776 |
| [4] |
Zhang, X.; Deng, B.; Fan, H.; Huang, W.; Zhang, Y. Acta Chim. Sinica. 2020, 78, 1120. (in Chinese)
doi: 10.6023/A20060230 |
|
(张旭寒, 邓博文, 范海东, 黄文辉, 张彦威, 化学学报, 2020, 78, 1120.)
doi: 10.6023/A20060230 |
|
| [5] |
Hoch, L. B.; O’Brien, P. G.; Jelle, A.; Sandhel, A.; Perovic, D. D.; Mims, C. A.; Ozin, G. A. ACS Nano 2016, 10, 9017.
doi: 10.1021/acsnano.6b05416 |
| [6] |
Wang, L.; Dong, Y.; Yan, T.; Hu, Z.; Jelle, A. A.; Meira, D. M.; Duchesne, P. N.; Loh, J. Y. Y.; Qiu, C.; Storey, E. E.; Xu, Y.; Sun, W.; Ghoussoub, M.; Kherani, N. P.; Helmy, A. S.; Ozin, G. A. Nat. Commun. 2020, 11, 2432.
doi: 10.1038/s41467-020-16336-z pmid: 32415078 |
| [7] |
Hoch, L. B.; O’Brien, P. G.; Jelle, A.; Sandhel, A.; Perovic, D. D.; Mims, C. A.; Ozin, G. A. ACS Nano 2016, 10, 9017.
doi: 10.1021/acsnano.6b05416 |
| [8] |
Li, Y. F.; Lu, W.; Chen, K.; Duchesne, P.; Jelle, A.; Xia, M.; Wood, T. E.; Ulmer, U.; Ozin, G. A. J. Am. Chem. Soc. 2019, 141, 14991.
doi: 10.1021/jacs.9b08030 |
| [9] |
Jia, J.; Wang, H.; Lu, Z.; O’Brien, P. G.; Ghoussoub, M.; Duchesne, P.; Zheng, Z.; Li, P.; Qiao, Q.; Wang, L.; Gu, A.; Jelle, A. A.; Dong, Y.; Wang, Q.; Ghuman, K. K.; Wood, T.; Qian, C.; Shao, Y.; Qiu, C.; Ye, M.; Zhu, Y.; Lu, Z. H.; Zhang, P.; Helmy, A. S.; Singh, C. V.; Kherani, N. P.; Perovic, D. D.; Ozin, G. A. Adv. Sci. 2017, 4, 1700252.
doi: 10.1002/advs.v4.10 |
| [10] |
Li, C.; Chen, F.; Ye, L.; Li, W.; Yu, H.; Zhao, T. Acta Chim. Sinica 2020, 78, 1448. (in Chinese)
doi: 10.6023/A20070322 |
|
(李宸, 陈凤华, 叶丽, 李伟, 于晗, 赵彤, 化学学报, 2020, 78, 1448.)
doi: 10.6023/A20070322 |
|
| [11] |
Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J. M.; Domen, K.; Antonietti, M. A. Nat. Mater. 2009, 8, 76.
doi: 10.1038/nmat2317 |
| [12] |
Ong, W. J.; Tan, L. L.; Ng, Y. H.; Yong, S. T.; Chai, S. P. Chem. Rev. 2016, 116, 7159.
doi: 10.1021/acs.chemrev.6b00075 |
| [13] |
Huang, Z. F.; Song, J.; Pan, L.; Wang, Z.; Zhang, X.; Zou, J. J.; Mi, W.; Zhang, X.; Wang, L. Nano Energy 2015, 12, 646.
doi: 10.1016/j.nanoen.2015.01.043 |
| [14] |
Chen, P.; Lei, B.; Dong, X.; Wang, H.; Sheng, J.; Cui, W.; Li, J.; Sun, Y.; Wang, Z.; Dong, F. ACS Nano 2020, 14, 15841.
doi: 10.1021/acsnano.0c07083 |
| [15] |
Chen, S.; Hu, Y.; Meng, S.; Fu, X. Appl. Catal., B 2014, 150-151, 564.
doi: 10.1016/j.apcatb.2013.12.053 |
| [16] |
Grosvenor, A. P.; Kobe, B. A.; Biesinger, M. C.; McIntyre, N. S. Surf. Interface Anal. 2004, 36, 1564.
doi: 10.1002/(ISSN)1096-9918 |
| [17] |
Palmer, J. W.; Swartz Jr, W. E.; King, D.; Stanko, J. A. Fla. Sci. 1993, 56, 123.
|
| [18] |
Romeo, M.; Majerus, J.; Legare, P.; Castellani, N. J.; Leroy, D. B. Surf. Sci. 1990, 238, 163.
doi: 10.1016/0039-6028(90)90073-H |
| [19] |
Liu, H. Y.; Chiou, W. A.; Fröhlich, G.; Sachtler, W. M. H. Top. Catal. 2000, 10, 49.
doi: 10.1023/A:1019103815248 |
| [20] |
Sun, M. H.; Huang, S. Z.; Chen, L. H.; Li, Y.; Yang, X. Y.; Yuan, Z. Y.; Su, B. L. Chem. Soc. Rev. 2016, 45, 3479.
doi: 10.1039/C6CS00135A |
| [21] |
He, S.; Li, W.; Wang, X.; Ma, Q.; Li, M.; Xu, W.; Wang, X.; Zhao, C. Appl. Surf. Sci. 2020, 506, 144948.
doi: 10.1016/j.apsusc.2019.144948 |
| [22] |
Kong, T.; Stolze, K.; Timmons, E. I.; Tao, J.; Ni, D.; Guo, S.; Yang, Z.; Prozorov, R.; Cava, R. J. Adv. Mater. 2019, 31, 1.
|
| [23] |
Wang, L.; Ghoussoub, M.; Wang, H.; Shao, Y.; Sun, W.; Tountas, A. A.; Wood, T. E.; Li, H.; Loh, J. Y. Y.; Dong, Y.; Xia, M.; Li, Y.; Wang, S.; Jia, J.; Qiu, C.; Qian, C.; Kherani, N. P.; He, L.; Zhang, X.; Ozin, G. A. Joule 2018, 2, 1369.
doi: 10.1016/j.joule.2018.03.007 |
| [24] |
Zhu, Z.; Lu, Z.; Wang, D.; Tang, X.; Yan, Y.; Shi, W.; Wang, Y.; Gao, N.; Yao, X.; Dong, H. Appl. Catal., B 2016, 182, 115.
|
| [25] |
Xu, Y.; Schoonen, M. A. A. Am. Mineral. 2000,543.
|
| [26] |
Wu, H. Z.; Liu, L. M.; Zhao, S. J. Phys. Chem. Chem. Phys. 2014, 16, 3299.
doi: 10.1039/c3cp54333a |
| [27] |
Moniz, S. J. A.; Shevlin, S. A.; Martin, D. J.; Guo, Z. X.; Tang, J. Energy Environ. Sci. 2015, 8, 731.
doi: 10.1039/C4EE03271C |
| [28] |
Arndt, B.; Creutzburg, M.; Grånäs, E.; Volkov, S.; Krausert, K.; Vlad, A.; Noei, H.; Stierle, A. J. Phys. Chem. C 2019, 123, 26662.
doi: 10.1021/acs.jpcc.9b07160 |
| [29] |
Mirabella, F.; Zaki, E.; Ivars-Barcelo, F.; Schauermann, S.; Shaikhutdinov, S.; Freund, H. J. J. Phys. Chem. C 2018, 122, 27433.
doi: 10.1021/acs.jpcc.8b08240 |
| [30] |
Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A. E.; Evans, J.; Senanayake, S. D.; Stacchiola, D. J.; Liu, P.; Hrbek, J.; Fernández Sanz, J.; Rodriguez, J. A. Science 2014, 345, 546.
doi: 10.1126/science.1253057 pmid: 25082699 |
| [31] |
Hakim, A.; Marliza, T. S.; Abu Tahari, N. M.; Wan Isahak, R. W. N.; Yusop, R. M.; Mohamed Hisham, W. M.; Yarmo, A. M. Ind. Eng. Chem. Res. 2016, 55, 7888.
doi: 10.1021/acs.iecr.5b04091 |
| [32] |
Gracia, F. J.; Bollmann, L.; Wolf, E. E.; Miller, J. T.; Kropf, A. J. J. Catal. 2003, 220, 382.
doi: 10.1016/S0021-9517(03)00296-3 |
| [33] |
Benziger, J. B.; Larson, L. R. J. Catal. 1982, 77, 550.
doi: 10.1016/0021-9517(82)90195-6 |
| [34] |
Li, X.; Lin, J.; Li, L.; Huang, Y.; Pan, X.; Collins, S. E.; Ren, Y.; Su, Y.; Kang, L.; Liu, X.; Zhou, Y.; Wang, H.; Wang, A.; Qiao, B.; Wang, X.; Zhang, T. Angew. Chem. Int. Ed. 2020, 59, 2.
|
| [35] |
Panayotov, D. A.; Yates, J. T. J. Phys. Chem. C 2007, 111, 2959.
doi: 10.1021/jp066686k |
| [36] |
Skomurski, F. N.; Kerisit, S.; Rosso, K. M. Geochim. Cosmochim. Acta 2010, 74, 4234.
doi: 10.1016/j.gca.2010.04.063 |
| [37] |
Parkinson, G. S.; Mulakaluri, N.; Losovyj, Y.; Jacobson, P.; Pentcheva, R.; Diebold, U. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 82, 125413.
doi: 10.1103/PhysRevB.82.125413 |
| [38] |
Novotny, Z.; Mulakaluri, N.; Edes, Z.; Schmid, M.; Pentcheva, R.; Diebold, U.; Parkinson, G. S. Phys. Rev. B: Condens. Matter Mater. Phys. 2013, 87, 195410.
doi: 10.1103/PhysRevB.87.195410 |
| [39] |
Kurahashi, M.; Sun, X.; Yamauchi, Y. Phys. Rev. B: Condens. Matter Mater. Phys. 2010, 81, 193402.
doi: 10.1103/PhysRevB.81.193402 |
| [40] |
Baltrusaitis, J.; Jensen, J. H.; Grassian, V. H. J. Phys. Chem. B 2006, 110, 12005.
doi: 10.1021/jp057437j |
| [41] |
Gálvez, M. E.; Loutzenhiser, P. G.; Hischier, I.; Steinfeld, A. Energy Fuels 2008, 22, 3544.
doi: 10.1021/ef800230b |
| [1] | 田茹心, 杨苗, 陈果, 刘豇汕, 袁梦梅, 原弘, 欧阳述昕, 张铁锐. 钌/石英滤纸: 可回收型CO2甲烷化光热催化膜★[J]. 化学学报, 2023, 81(8): 869-873. |
| [2] | 徐赫, 韩鹏博, 秦安军, 唐本忠. 光热材料的发展现状及应用前景★[J]. 化学学报, 2023, 81(10): 1420-1437. |
| [3] | 解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实. 石墨相氮化碳的共轭交联修饰及其对可见光催化产氢性能的影响[J]. 化学学报, 2022, 80(9): 1231-1237. |
| [4] | 李泽洋, 杨宇森, 卫敏. 二氧化碳还原电催化剂的结构设计及性能研究进展[J]. 化学学报, 2022, 80(2): 199-213. |
| [5] | 王金格, 周伟, 李佳轶, 丁雅妮, 高继慧. 脉冲电催化的研究进展及性能强化机制[J]. 化学学报, 2022, 80(11): 1555-1568. |
| [6] | 王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣. 石墨烯量子点/铁基金属-有机骨架复合材料高效光催化二氧化碳还原※[J]. 化学学报, 2022, 80(1): 22-28. |
| [7] | 马一宁, 施润, 张铁锐. 三相界面电催化二氧化碳还原研究进展[J]. 化学学报, 2021, 79(4): 369-377. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||