化学学报 ›› 2021, Vol. 79 ›› Issue (12): 1502-1510.DOI: 10.6023/A21080385 上一篇 下一篇
研究论文
高霞a,*( ), 潘会宾b, 贺曾贤a, 杨柯a, 乔成芳a, 刘永亮a, 周春生a
), 潘会宾b, 贺曾贤a, 杨柯a, 乔成芳a, 刘永亮a, 周春生a
投稿日期:2021-08-16
发布日期:2021-10-08
通讯作者:
高霞
基金资助:
Xia Gaoa( ), Huibin Panb, Zengxian Hea, Ke Yanga, Chengfang Qiaoa, Yongliang Liua, Chunsheng Zhoua
), Huibin Panb, Zengxian Hea, Ke Yanga, Chengfang Qiaoa, Yongliang Liua, Chunsheng Zhoua
Received:2021-08-16
Published:2021-10-08
Contact:
Xia Gao
Supported by:文章分享
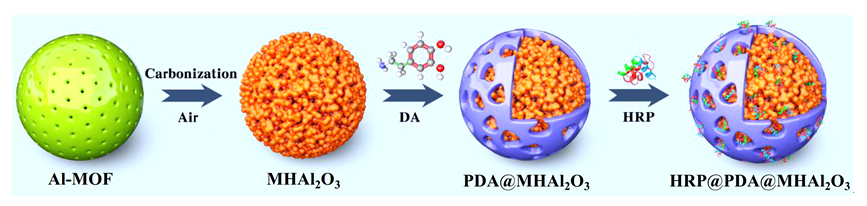
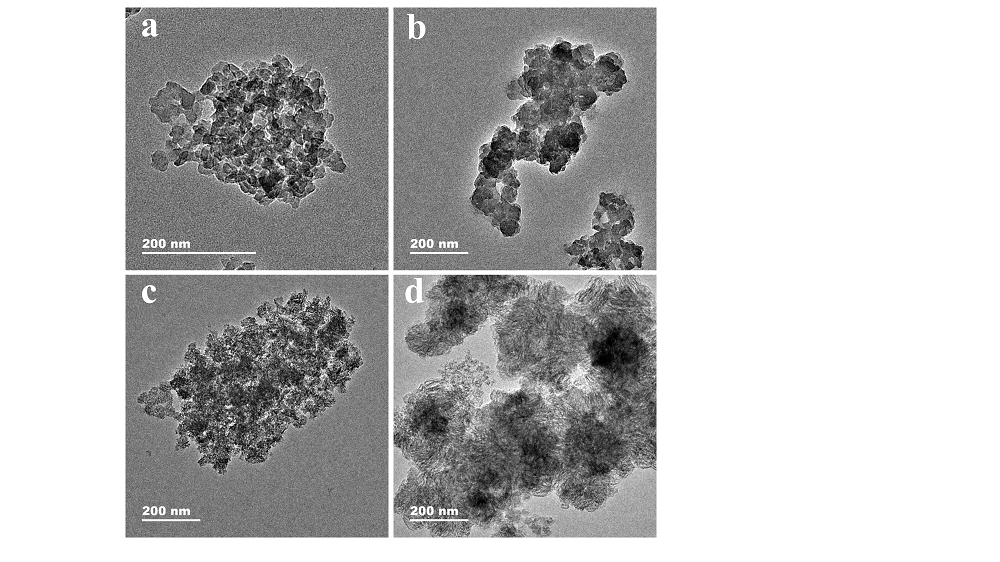
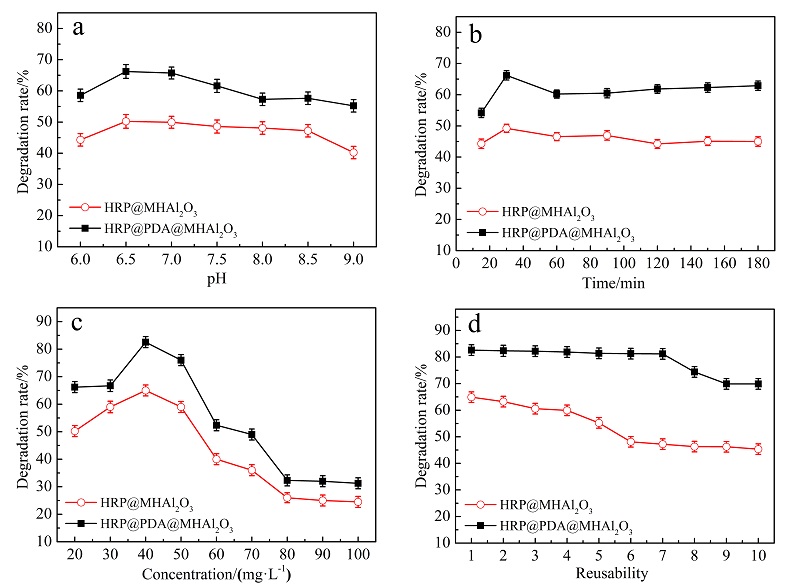
针对生物酶在固相载体负载后存在的催化活性与稳定性之间“此消彼长”的问题, 本工作采用“自牺牲模板”策略以铝基金属有机骨架材料(Al-MOF)为前驱体设计制备多级孔Al2O3 (MHAl2O3)材料, 再以“聚多巴胺(PDA)”仿生膜对材料表面进行功能化修饰后用以固载辣根过氧化物酶(HRP). 通过调节前驱体的煅烧温度来实现载体孔径大小的调控, 探讨了载体的孔道限域效应对固定化酶反应器催化活性的影响, 所得固定化酶反应器的热稳定性和重复使用性显著提高. 为了解析固定化酶反应器的构效关系, 采用酶动力学和热动力学参数研究了固定化酶反应器催化过程中酶与底物的相互作用, 结果表明固载后酶分子对底物的亲和性和专一性得到提升. 将固定化酶反应器用于模拟废水中苯胺黑药的催化降解时, 表现出非常高效的催化效率.
高霞, 潘会宾, 贺曾贤, 杨柯, 乔成芳, 刘永亮, 周春生. Al-MOF基多级孔Al2O3固定化酶反应器的构筑及构效关系研究[J]. 化学学报, 2021, 79(12): 1502-1510.
Xia Gao, Huibin Pan, Zengxian He, Ke Yang, Chengfang Qiao, Yongliang Liu, Chunsheng Zhou. Construction and Structure-Activity Relationship of Immobilized Enzyme Reactor Based on Al-MOF-Derived Al2O3 with Hierarchical Structure[J]. Acta Chimica Sinica, 2021, 79(12): 1502-1510.
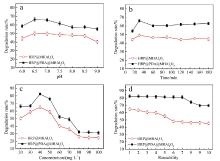
| Enzyme | vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(L•mmol-1•s-1) |
|---|---|---|---|---|
| Free HRP | 0.5355 | 0.4093 | 21.4200 | 52.3333 |
| HRP@MHAl2O3 | 1.1254 | 0.1125 | 45.0160 | 400.1422 |
| HRP@PDA@MHAl2O3 | 1.3456 | 0.0807 | 53.8240 | 666.9641 |
| Enzyme | vmax/(mmol•L-1•s-1) | Km/(mmol•L-1) | kcat/s-1 | (kcat/Km)/(L•mmol-1•s-1) |
|---|---|---|---|---|
| Free HRP | 0.5355 | 0.4093 | 21.4200 | 52.3333 |
| HRP@MHAl2O3 | 1.1254 | 0.1125 | 45.0160 | 400.1422 |
| HRP@PDA@MHAl2O3 | 1.3456 | 0.0807 | 53.8240 | 666.9641 |
| Enzyme | Ka×10-3/(L•mol-1) | ΔH/(kJ•mol-1) | ΔS/(J•mol-1•K-1) | n | TΔS/(kJ•mol-1) | ΔG/(kJ•mol-1) |
|---|---|---|---|---|---|---|
| Free HRP | 0.75±0.01 | 1088.0±0.01 | 3703.0±0.2 | 1.23±0.1 | 1104.0±0.1 | –16.41±0.04 |
| HRP@MHAl2O3 | 0.88±0.01 | 996.9±0.02 | 3409.0±0.1 | 1.36±0.1 | 1016.0±0.2 | –16.79±0.06 |
| HRP@PDA@MHAl2O3 | 1.03±0.01 | 142.2±0.01 | 534.4±0.1 | 6.91±0.1 | 159.3±0.2 | –17.19±0.06 |
| Enzyme | Ka×10-3/(L•mol-1) | ΔH/(kJ•mol-1) | ΔS/(J•mol-1•K-1) | n | TΔS/(kJ•mol-1) | ΔG/(kJ•mol-1) |
|---|---|---|---|---|---|---|
| Free HRP | 0.75±0.01 | 1088.0±0.01 | 3703.0±0.2 | 1.23±0.1 | 1104.0±0.1 | –16.41±0.04 |
| HRP@MHAl2O3 | 0.88±0.01 | 996.9±0.02 | 3409.0±0.1 | 1.36±0.1 | 1016.0±0.2 | –16.79±0.06 |
| HRP@PDA@MHAl2O3 | 1.03±0.01 | 142.2±0.01 | 534.4±0.1 | 6.91±0.1 | 159.3±0.2 | –17.19±0.06 |
| [1] |
Zhou, Y. G.; Mohamadi, R. M.; Poudineh, M.; Kermanshah, L.; Ahmed, S.; Safaei, T. S.; Stojcic, J.; Nam, R. K.; Sargent, E. H.; Kelley, S. O. Small 2016, 12, 727.
doi: 10.1002/smll.v12.6 |
| [2] |
Chen, W.; Jin, B.; Hu, Y. L.; Lu, Y.; Xia, X. H. Small 2012, 8, 1001.
doi: 10.1002/smll.201102117 pmid: 22311804 |
| [3] |
Cui, J. W.; He, S.; Dai, S.; Liu, L. Y.; Zhao, A.; Lu, L.; Yang, P.; Chen, J.; Huang, N. Chem. Eng. J. 2021, 424, 130392.
doi: 10.1016/j.cej.2021.130392 |
| [4] |
Cheng, H. P.; Hu, M. C.; Zhai, Q. G.; Li, S. N.; Jiang, Y. C. Chem. Eng. J. 2018, 347, 703.
doi: 10.1016/j.cej.2018.04.083 |
| [5] |
Zhuang, W.; Quan, X. B.; Wang, Z. F.; Zhou, W. F.; Yang, P. P.; Ge, L.; Hernandez, B. V.; Wu, J. L.; Li, M.; Zhou, J.; Zhu, C. J.; Ying, H. J. Chem. Eng. J. 2020, 394, 125038.
|
| [6] |
Sankaran, R.; Show, P. L.; Chang, J. S. Biofuel. Bioprod. Bior. 2016, 10, 896.
doi: 10.1002/bbb.2016.10.issue-6 |
| [7] |
Zhao, X. B.; Qi, F.; Yuan, C. L.; Du, W.; Liu, D. H. Renew. Sust. Energy Rev. 2015, 44, 182.
|
| [8] |
An, K. J.; Kwon, S. G.; Park, M.; Na, H. B.; Baik, S.; Yu, J. H.; Kim, D.; Son, J. S.; Kim, Y.W.; Song, I. C.; Moon, W. K.; Park, H. M.; Hyeon, T. Nano Lett. 2008, 8, 4252.
doi: 10.1021/nl8019467 |
| [9] |
Xiong, S. L.; Zeng, H. C. Angew. Chem. Int. Ed. 2012, 51, 949.
doi: 10.1002/anie.v51.4 |
| [10] |
Chen, J.; He, S. M.; Huang, B.; Zhang, L. Y.; Qiao, Z. Q.; Wang, J.; Yang, G. C.; Huang, H.; Hao, Q. L. Appl. Surf. Sci. 2018, 457, 508.
doi: 10.1016/j.apsusc.2018.06.301 |
| [11] |
Journet, C.; Maser, W. K.; Bernier, P.; Loiseau, A.; Lamy de la Chapelle, M.; Lefrant, S.; Deniard, P.; Lee, R.; Fischer, J. E. Nature 1997, 388, 756.
doi: 10.1038/41972 |
| [12] |
Ishaq, S.; Tamime, R.; Bilad, M. R.; Khan, A. L. Sep. Purif. Technol. 2019, 210, 442.
doi: 10.1016/j.seppur.2018.08.031 |
| [13] |
Deng, Q.; Wang, R. Catal. Commun. 2019, 120, 11.
doi: 10.1016/j.catcom.2018.11.009 |
| [14] |
Zhang, W. Q.; Li, Q. Y.; Yang, X. Y.; Ma, Z.; Wang, H. H.; Wang, X. J. Acta Chim. Sinica 2017, 75, 80 (in Chinese).
|
|
( 张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军, 化学学报, 2017, 75, 80.)
doi: 10.6023/A16090496 |
|
| [15] |
Small, L. J.; Hill, R. C.; Krumhansl, J. L.; Schindelholz, M. E.; Chen, Z.; Chapman, K. W.; Zhang, X.; Yang, S.; Schröder, M.; Nenoff, T. M. ACS Appl. Mater. Inter. 2019, 11, 27982.
doi: 10.1021/acsami.9b09938 |
| [16] |
Chang, Z.; Qiao, Y.; Yang, H. J.; Deng, H.; Zhu, X. Y.; He, P.; Zhou, H. S. Acta Chim. Sinica 2021, 79, 139 (in Chinese).
doi: 10.6023/A20090442 |
|
( 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎, 化学学报, 2021, 79, 139.)
doi: 10.6023/A20090442 |
|
| [17] |
Wu, M. X.; Yang, Y. W. Adv. Mater. 2017, 29, 1606134.
doi: 10.1002/adma.201606134 |
| [18] |
Chen, X. R.; Tong, R. L.; Shi, Z. Q.; Yang, B.; Liu, H.; Ding, S. P.; Wang, X.; Lei, Q. F.; Wu, J.; Fang, W. J. ACS. Appl. Mater. Inter. 2018, 10, 2328.
|
| [19] |
Liu, Y.; Shao, X. X.; Kong, D. Q.; Li, G. Q.; Li, Q. S. Colloids Surf. B Biointerfaces 2021, 197, 111450.
doi: 10.1016/j.colsurfb.2020.111450 |
| [20] |
Zhao, R. N.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Acta Chim. Sinica 2017, 75, 293 (in Chinese).
doi: 10.6023/A16110593 |
|
( 赵睿南, 胡满成, 李淑妮, 翟全国, 蒋育澄, 化学学报, 2017, 75, 293.)
doi: 10.6023/A16110593 |
|
| [21] |
Nadar, S.; Rathod, V. K. Int. J. Biol. Macromol. 2018, 120, 2293.
doi: 10.1016/j.ijbiomac.2018.08.126 |
| [22] |
Liu, X. P.; Yan, Z. Q.; Zhang, Y.; Liu, Z. W.; Sun, Y. H.; Ren, J. S.; Qu, X. G. ACS Nano 2019, 13, 5222.
doi: 10.1021/acsnano.8b09501 |
| [23] |
Yao, X. F.; Li, Y. W. Chin. Sci. Bull. 2015, 60, 1906 (in Chinese).
doi: 10.1360/N972015-00438 |
|
( 姚显芳, 李映伟, 科学通报, 2015, 60, 1906.)
|
|
| [24] |
Bhadra, B. N.; Vinu, A.; Serre, C.; Jhung, S. H. Mater. Today 2019, 25, 88.
doi: 10.1016/j.mattod.2018.10.016 |
| [25] |
Zhong, M.; Kong, L.; Li, N.; Liu, Y. Y.; Zhu, J.; Bu, X. H. Coord. Chem. Rev. 2019, 388, 172.
doi: 10.1016/j.ccr.2019.02.029 |
| [26] |
Salunkhe, R. R.; Kaneti, Y. V.; Yamauchi, Y. ACS Nano 2017, 11, 5293.
doi: 10.1021/acsnano.7b02796 pmid: 28613076 |
| [27] |
Wei, W. B.; Dong, S. Y.; Huang, G. Q.; Xie, Q.; Huang, T. L. Sens. Actuators B: Chem. 2018, 260, 189.
doi: 10.1016/j.snb.2017.12.207 |
| [28] |
Xie, W.; Zhou, L. J.; Xu, J.; Guo, Q. L.; Jiang, F. L.; Liu, Y. Acta Phys.-Chim. Sin. 2020, 36, 1905051 (in Chinese).
|
|
( 谢文, 周莲娇, 徐娟, 郭清莲, 蒋风雷, 刘义, 物理化学学报, 2020, 36, 1905051.)
|
|
| [29] |
Li, L.; Xiang, S. L.; Cao, S. Q.; Zhang, J. Y.; Ouyang, G. F.; Chen, L. P.; Su, C. Y. Nat. Commun. 2013, 4, 1774.
doi: 10.1038/ncomms2757 |
| [30] |
Chen, Y.; Xiao, Z.; Liu, Y.; Fan, L. Z. J. Mater. Chem. 2017, 5, 24178.
|
| [31] |
Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Song, J.; Jiang, Y. C. J. Chem. Technol. Biot. 2019, 94, 1249.
|
| [32] |
Song, Y. C.; Hu, M. C.; Li, S. N.; Zhai, Q. G.; Jiang, Y. C. Chem. J. Chin. Univ. 2019, 40, 1805 (in Chinese).
|
|
( 宋艺超, 胡满成, 李淑妮, 翟全国, 蒋育澄, 高等学校化学学报, 2019, 40, 1805.)
|
|
| [33] |
Yang, Y.; Wang, S.; Zhou, Z.; Zhang, R.; Shen, H.; Song, J.; Su, P.; Yang, Y. BioChem. Eng. J. 2018, 137, 108.
doi: 10.1016/j.bej.2018.05.019 |
| [34] |
Cao, D. L.; Cheng, W. J.; Tao, K.; Liang, Y. X. Macromol. Res. 2018, 26, 616.
doi: 10.1007/s13233-018-6087-z |
| [35] |
Xiang, L.; Xiao, T.; Mo, C. H.; Zhao, H. M.; Li, Y. W.; Li, H.; Cai, Q. Y.; Zhou, D. M.; Wong, M. H. Ecotoxicol. Environ. Saf. 2018, 154, 84.
doi: 10.1016/j.ecoenv.2018.01.032 |
| [36] |
Fu, P. F.; Ma, Y. H.; Lei, B. L.; Li, G.; Lin, X. F. Environ. Technol. 2021, 42, 659.
doi: 10.1080/09593330.2019.1642389 |
| [37] |
Gao, X.; Zhai, Q. G.; Hu, M. C.; Li, S. N.; Jiang, Y. C. Catal. Sci. Technol. 2021, 11, 2446.
doi: 10.1039/D0CY02146F |
| [1] | 王晓, 王星文, 肖乐辉. 单分子荧光成像研究单颗粒纳米催化机制[J]. 化学学报, 2023, 81(8): 1002-1014. |
| [2] | 王凯晴, 袁硕, 徐王东, 霍丹, 杨秋林, 侯庆喜, 于得海. ZIF-8@B-CNF复合气凝胶的制备及其吸附性能研究[J]. 化学学报, 2023, 81(6): 604-612. |
| [3] | 郑奉斌, 王琨, 林田, 王英龙, 李国栋, 唐智勇. 金属有机骨架封装金属纳米粒子复合材料的制备及其催化应用研究进展★[J]. 化学学报, 2023, 81(6): 669-680. |
| [4] | 高丰琴, 刘洋, 张引莉, 蒋育澄. 羧基功能化Fe3O4固定化酶反应器的构筑及性能研究[J]. 化学学报, 2023, 81(4): 338-344. |
| [5] | 苏东芮, 任小康, 于沄淏, 赵鲁阳, 王天宇, 闫学海. 酪氨酸衍生物调控酶催化路径可控合成功能黑色素★[J]. 化学学报, 2023, 81(11): 1486-1492. |
| [6] | 李小娟, 叶梓瑜, 谢书涵, 王永净, 王永好, 吕源财, 林春香. 氮氯共掺杂多孔碳活化过一硫酸盐降解苯酚的性能及机理研究[J]. 化学学报, 2022, 80(9): 1238-1249. |
| [7] | 牛犇, 翟振宇, 郝肖柯, 任婷莉, 李从举. 基于ZIF-8/PAN复合薄膜的柔性丙酮气体传感器[J]. 化学学报, 2022, 80(7): 946-955. |
| [8] | 刘芳, 潘婷婷, 任秀蓉, 鲍卫仁, 王建成, 胡江亮. HCDs@MIL-100(Fe)吸附剂的制备及其苯吸附性能研究[J]. 化学学报, 2022, 80(7): 879-887. |
| [9] | 陈俊敏, 崔承前, 刘瀚林, 李国栋. 金属有机框架与Pt粒子复合材料催化喹啉选择性加氢性能研究※[J]. 化学学报, 2022, 80(4): 467-475. |
| [10] | 王旭生, 杨胥, 陈春辉, 李红芳, 黄远标, 曹荣. 石墨烯量子点/铁基金属-有机骨架复合材料高效光催化二氧化碳还原※[J]. 化学学报, 2022, 80(1): 22-28. |
| [11] | 郭彩霞, 马小杰, 王博. 金属有机框架基复合材料的制备及其光热性能研究[J]. 化学学报, 2021, 79(8): 967-985. |
| [12] | 李旭飞, 闫保有, 黄维秋, 浮历沛, 孙宪航, 吕爱华. 金属有机骨架及其复合材料基于筛分复合效应的C2分离的研究进展[J]. 化学学报, 2021, 79(4): 459-471. |
| [13] | 张雅祺, 楚奇, 石勇, 高金索, 熊巍, 黄磊, 丁越. 双金属Ag-Ni-MOF-74的合成及低温CO催化还原NO性能研究[J]. 化学学报, 2021, 79(3): 361-368. |
| [14] | 谢佶晟, 肖竹梅, 左文华, 杨勇. 钠离子电池钴酸钠正极材料研究进展[J]. 化学学报, 2021, 79(10): 1232-1243. |
| [15] | 刘庆琳, 任保轶, 孙亚光, 解令海, 黄维. 螺芳基钙钛矿太阳能电池空穴传输材料研究进展[J]. 化学学报, 2021, 79(10): 1181-1196. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||