化学学报 ›› 2022, Vol. 80 ›› Issue (1): 16-21.DOI: 10.6023/A21100467 上一篇 下一篇
所属专题: 中国科学院青年创新促进会合辑
研究论文
李玲玲a,b, 刘宇a,b, 宋术岩a,b,*( ), 张洪杰a,b,c,*(
), 张洪杰a,b,c,*( )
)
投稿日期:2021-10-20
发布日期:2021-12-06
通讯作者:
宋术岩, 张洪杰
作者简介:基金资助:
Lingling Lia,b, Yu Liua,b, Shuyan Songa,b( ), Hongjie Zhanga,b,c(
), Hongjie Zhanga,b,c( )
)
Received:2021-10-20
Published:2021-12-06
Contact:
Shuyan Song, Hongjie Zhang
About author:Supported by:文章分享

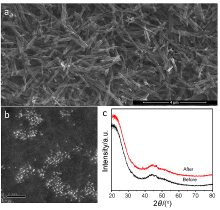
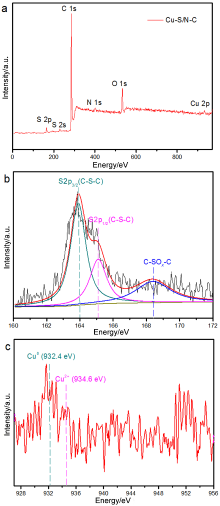
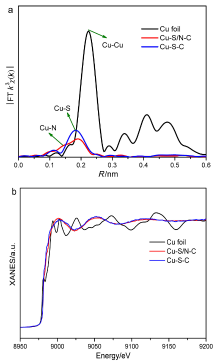
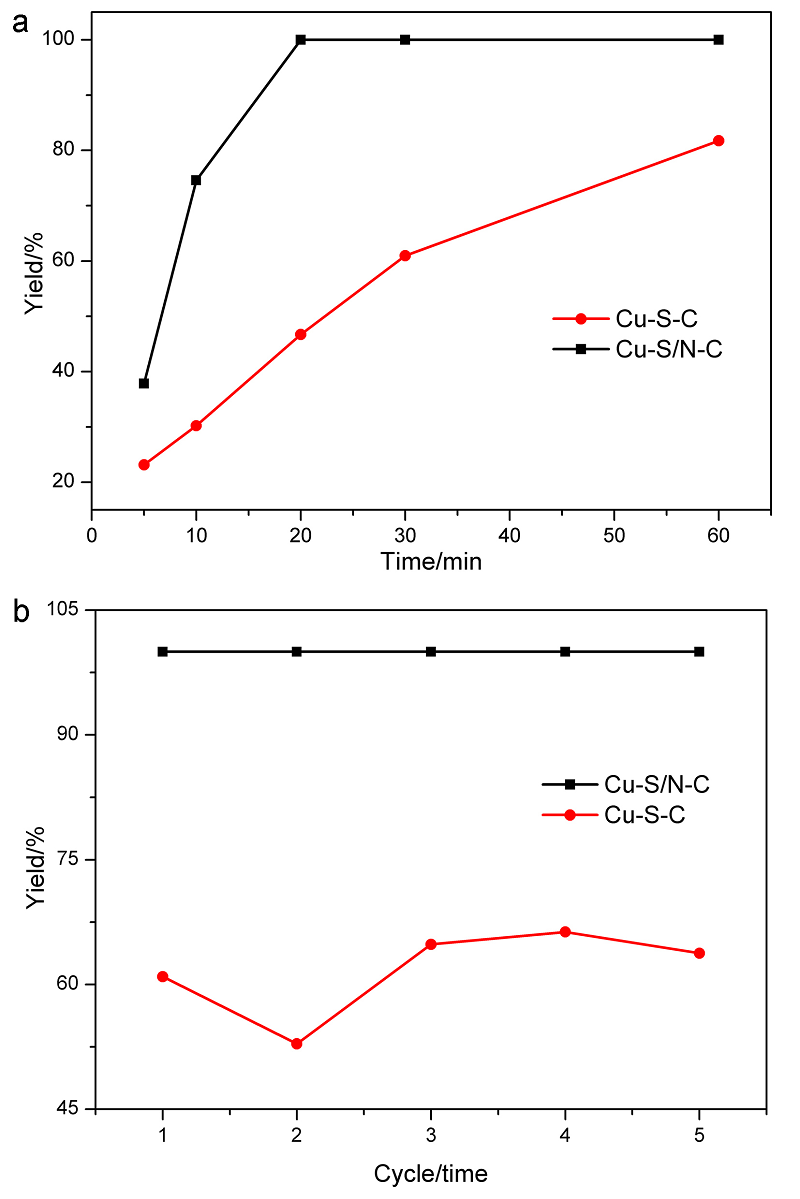
具有可控配位环境的高催化活性和选择性的稳定单金属位点催化剂的合成仍然具有挑战性. 本工作采用阳离子交换策略合成了两种具有不同配位结构的Cu单原子催化材料. 该策略主要依赖于硫化物的阴离子骨架和富含 N 的聚合物壳在高温退火过程中产生大量的S和N缺陷, 精确合成了富边缘S和N双修饰的单金属Cu位点催化材料. 在这两种材料, 一种Cu单原子具有硫(S)、氮(N)双配位, 一种Cu单原子只有单一的S配位. Cu中心原子的第一壳层配位数为4, Cu-S/N-C的结构为Cu-S1N3, Cu-S-C的结构为Cu-S4. 实验表明, S、N双修饰的Cu单原子材料在室温下催化硝基苯加氢过程中表现出较高活性. 反应20 min后, 在Cu-S/N-C催化下, 硝基苯加氢转化率达到100%, 循环使用5次后活性未见显著下降. 该发现为调节中心金属配位环境以提高单原子催化材料的性能提供了一种可行的方法.
李玲玲, 刘宇, 宋术岩, 张洪杰. 配位环境可调的Cu单原子的合成及催化加氢性能研究※[J]. 化学学报, 2022, 80(1): 16-21.
Lingling Li, Yu Liu, Shuyan Song, Hongjie Zhang. Synthesis of Cu Single Atom with Adjustable Coordination Environment and Its Catalytic Hydrogenation Performance※[J]. Acta Chimica Sinica, 2022, 80(1): 16-21.






| [1] |
Shuai, X. M.; Shen, W. Z. Nanoscale Res. Lett. 2012, 7, 1.
doi: 10.1186/1556-276X-7-1 |
| [2] |
Kundu, S.; Kundu, P.; Van Tendeloo, G.; Ravishankar, N. Small 2014, 10, 3895.
doi: 10.1002/smll.201400524 |
| [3] |
Zhang, H. B.; Pan, X. L.; Han, X. W.; Liu, X. M.; Wang, X. F.; Shen, W. L.; Bao, X. H. Chem. Sci. 2013, 4, 1075.
doi: 10.1039/c2sc21761a |
| [4] |
Wang, J.; Lei, M.; Wang, Z.; Liu, Y.; Zhuang, W.; Zhu, W. Appl. Surf. Sci. 2021, 542, 148541.
doi: 10.1016/j.apsusc.2020.148541 |
| [5] |
Cao, Y. H.; Guo, L.; Dan, M.; Doronkin, D. E.; Han, C. Q.; Rao, Z. Q.; Liu, Y.; Meng, J.; Huang, Z.; Zheng, K. B.; Chen, P.; Dong, F.; Zhou, Y. Nat. Commun. 2021, 12, 1675.
doi: 10.1038/s41467-021-21925-7 |
| [6] |
Wang, X.; Liu, D.; Song, S.; Zhang, H. J. Am. Chem. Soc. 2013, 135, 15864.
doi: 10.1021/ja4069134 |
| [7] |
Baskaran, S.; Xu, C.-Q.; Wang, Y.-G.; Garzón, I. L.; Li, J. Sci. China Mater. 2020, 63, 993.
doi: 10.1007/s40843-019-1257-x |
| [8] |
Li, F.; Li, Y.; Zeng, X. C.; Chen, Z. ACS Catal. 2014, 5, 544.
doi: 10.1021/cs501790v |
| [9] |
Wang, Q. S.; Zhang, Z. S.; Wang, H.; Liu, Y.; Song, S. Y.; Zhang, H. J. Chem. J. Chin. Univ. 2020, 41, 947. (in Chinese)
|
|
( 王启舜, 张泽树, 王欢, 刘宇, 宋术岩, 张洪杰, 高等学校化学学报, 2020, 41, 947.)
|
|
| [10] |
Wang, F.; Song, S. Y.; Li, K.; Li, J. Q.; Pan, J.; Yao, S.; Ge, X.; Feng, J.; Wang, X.; Zhang, H. J. Adv. Mater. 2016, 28, 10679.
doi: 10.1002/adma.201603608 |
| [11] |
Zhou, H.; Zhao, Y.; Gan, J.; Xu, J.; Wang, Y.; Lv, H.; Fang, S.; Wang, Z.; Deng, Z.; Wang, X.; Liu, P.; Guo, W.; Mao, B.; Wang, H.; Yao, T.; Hong, X.; Wei, S.; Duan, X.; Luo, J.; Wu, Y. J. Am. Chem. Soc. 2020, 142, 12643.
doi: 10.1021/jacs.0c03415 |
| [12] |
Deng, Z. J.; Ouyang, Y. F.; Ao, Y. L.; Cai, Q. Acta Chim. Sinica 2021, 79, 649. (in Chinese)
doi: 10.6023/A21010006 |
|
( 邓卓基, 欧阳溢凡, 敖运林, 蔡倩, 化学学报 2021, 79, 649.)
|
|
| [13] |
Fei, H. L.; Dong, J. C.; Arellano-Jimenez, M. J.; Ye, G. L.; Kim, N. D.; Samuel, E. L. G.; Peng, Z. W.; Zhu, Z.; Qin, F.; Bao, J. M.; Yacaman, M. J.; Ajayan, P. M.; Chen, D. L.; Tour, J. M. Nat. Commun. 2015, 6, 8.
|
| [14] |
Wan, J. W.; Chen, W. X.; Jia, C. Y.; Zheng, L. R.; Dong, J. C.; Zheng, X. S.; Wang, Y.; Yan, W. S.; Chen, C.; Peng, Q.; Wang, D. S.; Li, Y. D. Adv. Mater. 2018, 30, 8.
|
| [15] |
Wan, X.; Liu, X. F.; Li, Y. C.; Yu, R. H.; Zheng, L. R.; Yan, W. S.; Wang, H.; Xu, M.; Shui, J. L. Nat. Catal. 2019, 2, 259.
doi: 10.1038/s41929-019-0237-3 |
| [16] |
Jin, H. H.; Zhou, H.; Ji, P. X.; Zhang, C. T.; Luo, J. H.; Zeng, W. H.; Hu, C. X.; He, D. P.; Mu, S. C. Nano Res. 2020, 13, 818.
doi: 10.1007/s12274-020-2702-3 |
| [17] |
Hu, M. Y.; Li, S. N.; Zheng, S. S.; Liang, X. H.; Zheng, J. X.; Pan, F. J. Phys. Chem. C 2020, 124, 13168.
doi: 10.1021/acs.jpcc.0c01998 |
| [18] |
Li, L. L.; Chang, X.; Lin, X. Y.; Zhao, Z. J.; Gong, J. L. Chem. Soc. Rev. 2020, 49, 8156.
doi: 10.1039/D0CS00795A |
| [19] |
Chen, G. B.; Wang, T.; Liu, P.; Liao, Z. Q.; Zhong, H. X.; Wang, G.; Zhang, P. P.; Yu, M. H.; Zschech, E.; Chen, M. W.; Zhang, J.; Feng, X. L. Energ. Environ. Sci. 2020, 13, 2849.
doi: 10.1039/D0EE01613F |
| [20] |
Lu, X. Q.; Cao, S. F.; Wei, X. F.; Li, S. R.; Wei, S. X. Acta Chim. Sinica 2020, 78, 1001. (in Chinese)
doi: 10.6023/A20060223 |
|
( 鲁效庆, 曹守福, 魏晓飞, 李邵仁, 魏淑贤, 化学学报 2020, 78, 1001.)
|
|
| [21] |
Chen, Y. J.; Gao, R.; Ji, S. F.; Li, H. J.; Tang, K.; Jiang, P.; Hu, H. B.; Zhang, Z. D.; Hao, H. G.; Qu, Q. Y.; Liang, X.; Chen, W. X.; Dong, J. C.; Wang, D. S.; Li, Y. D. Angew. Chem. Int. Ed. 2020, 60, 3212.
doi: 10.1002/anie.v60.6 |
| [22] |
Chen, K. J.; Liu, K.; An, P. D.; Li, H. J. W.; Lin, Y. Y.; Hu, J. H.; Jia, C. K.; Fu, J. W.; Li, H. M.; Liu, H.; Lin, Z.; Li, W. Z.; Li, J. H.; Lu, Y. R.; Chan, T. S.; Zhang, N.; Liu, M. Nat. Commun. 2020, 11, 4173.
doi: 10.1038/s41467-020-18062-y |
| [23] |
Zhu, J.; Ren, Z.; Du, S.; Xie, Y.; Wu, J.; Meng, H.; Xue, Y.; Fu, H. Nano Res. 2017, 10, 1819-1831.
doi: 10.1007/s12274-017-1511-9 |
| [24] |
Jia, Y.; Jiang, K.; Wang, H.; Yao, X. Chem. 2019, 5, 1371-1397.
doi: 10.1016/j.chempr.2019.02.008 |
| [25] |
Wang, Y. S.; Zhao, Y. L.; Zhao, Z. Z.; Lan, X. L.; Xu, J. X.; Xu, W. X.; Duan, Z. K. Acta Chim. Sinica 2019, 77, 661. (in Chinese)
doi: 10.6023/A19040124 |
|
( 王永胜, 赵云鹭, 赵珍珍, 兰小林, 徐金霞, 徐伟祥, 段正康, 化学学报 2019, 77, 661.)
|
|
| [26] |
Shang, H. S.; Zhou, X. G.; Dong, J. C.; Li, A.; Zhao, X.; Liu, Q. H.; Lin, Y.; Pei, J. J.; Li, Z.; Jiang, Z. L.; Zhou, D. N.; Zheng, L. R.; Wang, Y.; Zhou, J.; Yang, Z. K.; Cao, R.; Sarangi, R.; Sun, T. T.; Yang, X.; Zheng, X. S.; Yan, W. S.; Zhuang, Z. B.; Li, J.; Chen, W. X.; Wang, D. S.; Zhang, J. T.; Li, Y. D. Nat. Commun. 2020, 11, 3049.
doi: 10.1038/s41467-020-16848-8 |
| [27] |
Li, Y.; Cheng, W.; Su, H.; Zhao, X.; He, J.; Liu, Q. Nano Energy 2020, 77, 105121.
doi: 10.1016/j.nanoen.2020.105121 |
| [28] |
Zhang, J.; Zhao, Y.; Chen, C.; Huang, Y.-C.; Dong, C.-L.; Chen, C.-J.; Liu, R.-S.; Wang, C.; Yan, K.; Li, Y.; Wang, G. J. Am. Chem. Soc. 2019, 141, 20118.
doi: 10.1021/jacs.9b09352 |
| [29] |
Che, M. Catal. Today 2013, 218, 162.
|
| [30] |
Ramaswamy, N.; Tylus, U.; Jia, Q.; Mukerjee, S. J. Am. Chem. Soc. 2013, 135, 15443.
doi: 10.1021/ja405149m |
| [31] |
Shang, H.; Sun, W.; Sui, R.; Pei, J.; Zheng, L.; Dong, J.; Jiang, Z.; Zhou, D.; Zhuang, Z.; Chen, W.; Zhang, J.; Wang, D.; Li, Y. Nano Lett. 2020, 20, 5443.
doi: 10.1021/acs.nanolett.0c01925 |
| [32] |
Li, X.; Bi, W.; Chen, M.; Sun, Y.; Ju, H.; Yan, W.; Zhu, J.; Wu, X.; Chu, W.; Wu, C.; Xie, Y. J. Am. Chem. Soc. 2017, 139, 14889.
doi: 10.1021/jacs.7b09074 |
| [33] |
Chen, J. Y.; Li, H.; Fan, C. A.; Meng, Q. W.; Tang, Y. W.; Qiu, X. Y.; Fu, G. T.; Ma, T. Y. Adv. Mater. 2020, 32, 2003134.
doi: 10.1002/adma.v32.30 |
| [34] |
Sun, Y.; Silvioli, L.; Sahraie, N. R.; Ju, W.; Li, J.; Zitolo, A.; Li, S.; Bagger, A.; Arnarson, L.; Wang, X.; Moeller, T.; Bernsmeier, D.; Rossmeisl, J.; Jaouen, F.; Strasser, P. J. Am. Chem. Soc. 2019, 141, 12372.
doi: 10.1021/jacs.9b05576 |
| [1] | 张晓萌, 李希雅, 熊晚枫, 李红芳, 曹荣. 基于超分子晶体制备超细铂纳米颗粒用于催化加氢硝基苯[J]. 化学学报, 2021, 79(2): 180-185. |
| [2] | 张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军. 含苯并噻二唑结构单元的金属有机骨架在可见光催化需氧氧化反应中的应用[J]. 化学学报, 2017, 75(1): 80-85. |
| [3] | 杨旭石, 黄建林, 朱凤霞, 李和兴. 乙基桥联有序介孔有机硅负载Pd(II)有机金属催化剂用于水介质Barbier反应[J]. 化学学报, 2010, 68(03): 217-221. |
| [4] | 尹安远 郭秀英 戴维林 范康年. 新型高性能Cu/HMS催化剂的合成及其在草酸二甲酯催化加氢合成 乙二醇反应中的应用[J]. 化学学报, 2009, 67(15): 1731-1736. |
| [5] | 徐华龙, 黄静静, 杨新艳, 杜俊明, 沈江, 沈伟. K-MnO/γ-Al2O3和Cu/SiO2催化剂应用于苯甲酸甲酯连续加氢合成无氯苯甲醇[J]. 化学学报, 2006, 64(16): 1615-1621. |
| [6] | 赵永祥,武志刚,许临萍,张临卿,刘滇生,徐贤伦. 前驱物对NiO/SiO_2气凝胶催化剂性能的影响[J]. 化学学报, 2002, 60(4): 596-599. |
| [7] | 沈百荣,方志刚,范康年,邓景发. Ni-Co-B非晶态合金的结构和催化活性的理论研究[J]. 化学学报, 1999, 57(4): 366-371. |
| [8] | 王多禄,张泽朋,陈立功,许正双,王以,黄红梅,孟Yi. 哌啶酮催化氢化制备哌啶醇催化剂的研究[J]. 化学学报, 1999, 57(2): 176-182. |
| [9] | 陈海鹰,邓景发,盛世善,陈恒荣,熊国兴. 非晶态金属合金作催化材料的研究V. Ni-W-P非晶态合金微粒催化加氢活性研究[J]. 化学学报, 1994, 52(9): 877-882. |
| [10] | 杨军,邓景发,董树忠. 非晶态金属合金作催化材料的研究 III. Ni-Fe-P非晶态合金对CO加氢活性的研究[J]. 化学学报, 1991, 49(9): 833-838. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||