化学学报 ›› 2022, Vol. 80 ›› Issue (1): 11-15.DOI: 10.6023/A21100457 上一篇 下一篇
研究通讯
杨民a,b, 叶柏柏b, 陈健强a,*( ), 吴劼a,*(
), 吴劼a,*( )
)
投稿日期:2021-10-13
发布日期:2021-12-06
通讯作者:
陈健强, 吴劼
基金资助:
Min Yanga,b, Baibai Yeb, Jianqiang Chena( ), Jie Wua(
), Jie Wua( )
)
Received:2021-10-13
Published:2021-12-06
Contact:
Jianqiang Chen, Jie Wu
Supported by:文章分享
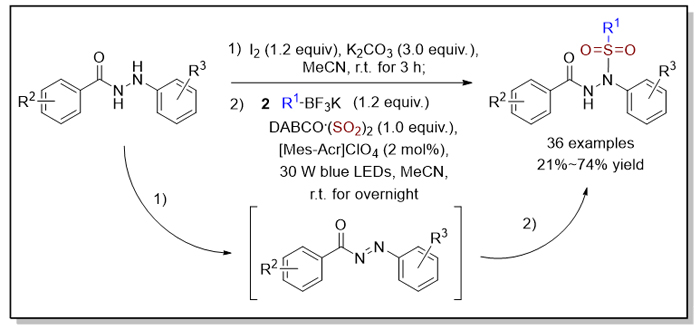
鉴于磺酰基在有机分子中的重要意义, 磺酰基的引入与磺酰化合物的合成被广泛报道. 其中, 磺酰肼化合物因在抗肿瘤、抗菌等方面表现出的生物活性, 其相关合成备受关注. 本工作发展了可见光照射下烷基三氟硼酸钾、DABCO•(SO2)2 (1,4-Diazabicyclo[2.2.2]octane, DABCO)和芳酰肼的有机光反应, 一锅法获得了中等到良好收率的N'-酰基-N-磺酰肼化合物, 该反应底物适用性广、绿色温和. 机理研究表明该反应经历自由基反应历程, 即由烷基三氟硼酸钾在光催化作用下原位生成的烷基自由基引发的, 随后通过二氧化硫插入形成烷基磺酰基自由基中间体, 最后被芳酰肼氧化产生的N-酰基二氮烯中间体捕获, 从而生成N'-酰基-N-磺酰化产物.
杨民, 叶柏柏, 陈健强, 吴劼. 可见光催化烷基磺酰自由基启动芳酰肼的烷基磺酰化反应[J]. 化学学报, 2022, 80(1): 11-15.
Min Yang, Baibai Ye, Jianqiang Chen, Jie Wu. Visible-light Photocatalytic Alkylsulfonylation of Aroylhydrazides with Alkylsulfonyl Radicals[J]. Acta Chimica Sinica, 2022, 80(1): 11-15.
| Entry | Photocatalyst (PC) | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | [Mes-Acr]ClO4 | DCE | 64% |
| 2 | Eosin Y | DCE | 6% |
| 3 | Ru(bpy)3Cl2 | DCE | 14% |
| 4 | Ir(ppy)3 | DCE | 8% |
| 5 | Rhodamine 6G | DCE | 4% |
| 6 | 4-CzIPN | DCE | trace |
| 7 | [Mes-Acr]ClO4 | MeCN | 73% |
| 8 | [Mes-Acr]ClO4 | THF | 47% |
| 9 | [Mes-Acr]ClO4 | CHCl3 | 70% |
| 10 | [Mes-Acr]ClO4 | DMF | 54% |
| 11 | [Mes-Acr]ClO4 | 1,4-dioxane | 55% |
| 12 | [Mes-Acr]ClO4 | MeOH | 10% |
| 13 | [Mes-Acr]ClO4 | EA | 71% |
| 14 c | [Mes-Acr]ClO4 | MeCN | 75% (74%) |
| Entry | Photocatalyst (PC) | Solvent | Yieldb/% |
|---|---|---|---|
| 1 | [Mes-Acr]ClO4 | DCE | 64% |
| 2 | Eosin Y | DCE | 6% |
| 3 | Ru(bpy)3Cl2 | DCE | 14% |
| 4 | Ir(ppy)3 | DCE | 8% |
| 5 | Rhodamine 6G | DCE | 4% |
| 6 | 4-CzIPN | DCE | trace |
| 7 | [Mes-Acr]ClO4 | MeCN | 73% |
| 8 | [Mes-Acr]ClO4 | THF | 47% |
| 9 | [Mes-Acr]ClO4 | CHCl3 | 70% |
| 10 | [Mes-Acr]ClO4 | DMF | 54% |
| 11 | [Mes-Acr]ClO4 | 1,4-dioxane | 55% |
| 12 | [Mes-Acr]ClO4 | MeOH | 10% |
| 13 | [Mes-Acr]ClO4 | EA | 71% |
| 14 c | [Mes-Acr]ClO4 | MeCN | 75% (74%) |
| [1] |
(a) Prinsep, M. R.; Blunt, J. W.; Munro, M. H. G. J. Nat. Prod. 1991, 54, 1068.
doi: 10.1021/np50076a023 |
|
(b) Teall, M.; Oakley, P.; Harrison, T. Bioorg. Med. Chem. Lett. 2005, 15, 2685.
doi: 10.1016/j.bmcl.2004.12.017 |
|
|
(c) Legros, L.; Dehli, J. R.; Bolm, C. Adv. Synth. Catal. 2005, 347, 19.
doi: 10.1002/(ISSN)1615-4169 |
|
|
(d) Churcher, I.; Beher, D.; Best, J. D. Bioorg. Med. Chem. Lett. 2006, 16, 280.
doi: 10.1016/j.bmcl.2005.10.009 |
|
|
(e) Harrak, Y.; Casula, G.; Basset, J. J. Med. Chem. 2010, 53, 6560.
doi: 10.1021/jm100398z |
|
| [2] |
Simpkins, N. S. Sulfones in Organic Synthesis, Pergamon Press, Oxford, 1993.
|
| [3] |
(a) Hayakawa, M.; Kaizawa, H.; Kawaguchi, K. I.; Ishikawa, N.; Koizumi, T.; Ohishi, T.; Workman, P. Bioorg. Med. Chem. 2007, 15, 403.
doi: 10.1016/j.bmc.2006.09.047 |
|
(b) Kamal, A.; Khan, M. N. A.; Reddy, K. S.; Rohini, K. Bioorg. Med. Chem. 2007, 15, 1004.
doi: 10.1016/j.bmc.2006.10.027 |
|
|
(c) Kendall, J. D.; Rewcastle, G. W.; Frederick, R.; Mawson, C.; Denny, W. A.; Marshall, E. S.; Shepherd, P. R. Bioorg. Med. Chem. 2007, 15, 7677.
doi: 10.1016/j.bmc.2007.08.062 |
|
|
(d) Riaz, S.; Khan, I. U.; Bajda, M.; Ashraf, M.; Shaukat, A.; Rehman, T. U.; Yar, M. Bioorg. Chem. 2015, 63, 64.
doi: 10.1016/j.bioorg.2015.09.008 |
|
|
(e) Wu, G.; Chen, R.; Yang, K.; Liu, H.; Wang, C. Chem. Res. Appl. 2020, 32, 1053. (in Chinese)
|
|
|
( 吴桂贞, 陈任宏, 杨凯, 刘海, 汪朝阳, 化学研究与应用, 2020, 32, 1053)
|
|
| [4] |
(a) Zheng, D.; An, Y.; Li, Z.; Wu, J. Angew. Chem. Int. Ed. 2014, 53, 2451.
doi: 10.1002/anie.201309851 |
|
(b) Namba, K.; Takeuchi, K.; Kaihara, Y.; Oda, M.; Nakayama, A.; Nakayama, A.; Tanino, K. Nat. Commun. 2015, 6, 1.
|
|
|
(c) Huang, Z.; Wang, C.; Dong, G. Angew. Chem. Int. Ed. 2016, 55, 5299.
doi: 10.1002/anie.201600912 |
|
|
(d) Sun, Q.; Li, L.; Liu, L.; Guan, Q.; Yang, Y.; Zha, Z.; Wang, Z. Org. Lett. 2018, 20, 5592.
doi: 10.1021/acs.orglett.8b02268 |
|
| [5] |
(a) Wang, Y.; Wang, H.; Liu, Z. Acta Chim. Sinica 2021, 79, 1085. (in Chinese)
doi: 10.6023/A21040179 |
|
( 王也铭, 王宏伟, 刘兆洪, 化学学报 2021, 79, 1085.)
|
|
|
(b) Ferwanah, A.-R.; Awadallah, A. Molecules 2005, 10, 492.
doi: 10.3390/10020492 |
|
|
(c) Sun, J.; Qiu, J.-K.; Zhu, Y.-L.; Guo, C.; Hao, W.-J.; Jiang, B.; Tu, S.-J. J. Org. Chem. 2015, 80, 8217.
doi: 10.1021/acs.joc.5b01280 |
|
|
(d) Sun, J.; Qiu, J.-K.; Jiang, B.; Hao, W.-J.; Guo, C.; Tu, S.-J. J. Org. Chem. 2016, 81, 3321.
doi: 10.1021/acs.joc.6b00332 |
|
|
(e) Sun, K.; Wang, X.; Fu, F.; Zhang, C.; Chen, Y.; Liu, L. Green Chem. 2017, 19, 1490.
doi: 10.1039/C6GC03420A |
|
|
(f) Wan, X.; Sun, K.; Zhang, G. Sci. China: Chem. 2017, 60, 353.
doi: 10.1007/s11426-016-0284-2 |
|
|
(g) Sun, K.; Lv, Y.; Shi, Z.; Fu, F.; Zhang, C.; Zhang, Z. Sci. China: Chem. 2017, 60, 730.
doi: 10.1007/s11426-016-0412-0 |
|
|
(h) Yang, Y.; Bao, Y. J.; Guan, Q. Q.; Sun, Q.; Zha, Z. G.; Wang, Z. Y. Green Chem. 2017, 19, 112.
doi: 10.1039/C6GC03142K |
|
| [6] |
(a) Merino, E. Chem. Soc. Rev. 2011, 40, 3835.
doi: 10.1039/c0cs00183j |
|
(b) Rosenbaum, C.; Waldmann, H. Tetrahedron Lett. 2001, 42, 5677.
doi: 10.1016/S0040-4039(01)01075-9 |
|
|
(c) White, E. H.; Field, K. W.; Hendrickson, W. H.; Dzadzic, P.; Roswell, D. F.; Paik, S.; Mullen, P. W. J. Am. Chem. Soc. 1992, 114, 8023.
doi: 10.1021/ja00047a009 |
|
|
(d) Millington, C. R.; Quarrell, R.; Lowe, G. Tetrahedron Lett. 1998, 39, 7201.
doi: 10.1016/S0040-4039(98)01543-3 |
|
| [7] |
(a) Bandara, H. M. D.; Burdette, S. C. Chem. Soc. Rev. 2012, 41, 1809.
doi: 10.1039/C1CS15179G |
|
(b) Tsupova, S.; Meorg, U. Heterocycles 2014, 88, 129.
doi: 10.3987/REV-13-SR(S)3 |
|
| [8] |
(a) Waring, D. R.; Hallas, G. The chemistry and application of dyes, Plenum, New York, 1990.
|
|
(b) Hunger, K. Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH, Weinheim, 2003.
|
|
| [9] |
(a) Yoneda, F.; Suzuki, K.; Nitta, Y. J. Am. Chem. Soc. 1966, 88, 2328.
doi: 10.1021/ja00962a051 |
|
(b) Yoneda, F.; Suzuki, K.; Nitta, Y. J. Org. Chem. 1967, 32, 727.
doi: 10.1021/jo01278a049 |
|
|
(c) Cao, H. T.; Grée, R. Tetrahedron Lett. 2009, 50, 1493.
doi: 10.1016/j.tetlet.2009.01.080 |
|
|
(d) Stone, M. T. Org. Lett. 2011, 13, 2326.
doi: 10.1021/ol200579a |
|
|
(e) Bøgevig, A.; Juhl, K.; Kumaragurubaran, N.; Zhuang, W.; Jørgensen, K. A. Angew. Chem. Int. Ed. 2002, 41, 1790.
doi: 10.1002/(ISSN)1521-3773 |
|
|
(f) Liu, T.-Y.; Cui, H.-L.; Zhang, Y.; Jiang, K.; Du, W.; He, Z.-Q.; Chen, Y.-C. Org. Lett. 2007, 9, 3671.
doi: 10.1021/ol701648x |
|
|
(g) Cheng, L.; Liu, L.; Wang, D.; Chen, Y.-J. Org. Lett. 2009, 11, 3874.
doi: 10.1021/ol901405r |
|
|
(h) Lan, Q.; Wang, X.; He, R.; Ding, C.; Maruoka, K. Tetrahedron Lett. 2009, 50, 3280.
doi: 10.1016/j.tetlet.2009.02.041 |
|
|
(i) Dick, A. R.; Hull, K. L.; Sanford, M. S. J. Am. Chem. Soc. 2004, 126, 2300.
doi: 10.1021/ja031543m |
|
|
(j) Sharma, S.; Han, S. H.; Han, S.; Ji, W.; Oh, J.; Lee, S.-Y.; Oh, J. S.; Jung, Y. H.; Kim, I. S. Org. Lett. 2015, 17, 2852.
doi: 10.1021/acs.orglett.5b01298 |
|
| [10] |
(a) Forchiassin, M.; Risaliti, A.; Russo, C. Tetrahedron 1981, 37, 2921.
doi: 10.1016/S0040-4020(01)92366-X |
|
(b) Chan, A.; Scheidt, K. A. J. Am. Chem. Soc. 2008, 130, 2740.
doi: 10.1021/ja711130p |
|
|
(c) Huang, X.-L.; He, L.; Shao, P.-L.; Ye, S. Angew. Chem. Int. Ed. 2009, 48, 192.
|
|
|
(d) Yang, L.; Wang, F.; Lee, R.; Lv, Y.; Huang, K.-W.; Zhong, G. Org. Lett. 2014, 16, 3872.
doi: 10.1021/ol501424f |
|
|
(e) Morrill, L. C.; Lebl, T.; Slawin, A. M. Z.; Smith, A. D. Chem. Sci. 2012, 3, 2088.
doi: 10.1039/c2sc20171b |
|
|
(f) Zhang, Q.; Meng, L.-G.; Zhang, J.; Wang, L. Org. Lett. 2015, 17, 3272.
doi: 10.1021/acs.orglett.5b01237 |
|
|
(g) Savva, A. C.; Mirallai, S. I.; Zissimou, G. A.; Berezin, A. A.; Demetriades, M.; Kourtellaris, A.; Constantinides, C. P.; Nicolaides, C.; Trypiniotis, T.; Koutentis, P. A. J. Org. Chem. 2017, 82, 7564.
doi: 10.1021/acs.joc.7b01297 |
|
|
(h) Zhou, R.; Han, L.; Zhang, H.; Liu, R.; Li, R. Adv. Synth. Catal. 2017, 359, 3977.
doi: 10.1002/adsc.v359.22 |
|
|
(i) Ma, C.; Zhou, J.-Y.; Zhang, Y.-Z.; Mei, G.-J.; Shi, F. Angew. Chem. Int. Ed. 2018, 57, 5398.
doi: 10.1002/anie.v57.19 |
|
| [11] |
(a) Bisseret, P.; Blanchard, N. Org. Biomol. Chem. 2013, 11, 5393.
doi: 10.1039/c3ob40997j |
|
(b) Deeming, A. S.; Emmett, E. J.; Richards-Taylor, C. S.; Willis, M. C. Synthesis, 2014, 46, 2701.
doi: 10.1055/s-00000084 |
|
|
(c) Liu, G.; Fan, C.; Wu, J. Org. Biomol. Chem. 2015, 13, 1592.
doi: 10.1039/C4OB02139H |
|
|
(d) Emmett, E. J.; Willis, M. C. Asian J. Org. Chem. 2015, 4, 602.
doi: 10.1002/ajoc.201500103 |
|
|
(e) Zheng, D.; Wu, J. Sulfur Dioxide Insertion Reactions for Organic Synthesis, Nature Springer, Berlin, 2017.
|
|
|
(f) Qiu, G.; Zhou, K.; Gao, L.; Wu, J. Org. Chem. Front. 2018, 5, 691.
doi: 10.1039/C7QO01073G |
|
|
(g) Hofman, K.; Liu, N. W.; Manolikakes, G. Chem. Eur. J. 2018, 24, 11852.
doi: 10.1002/chem.v24.46 |
|
|
(h) Qiu, G.; Lai, L.; Cheng, J.; Wu, J. Chem. Commun. 2018, 54, 10405.
doi: 10.1039/C8CC05847D |
|
|
(i) Qiu, G.; Zhou, K.; Wu, J. Chem. Commun. 2018, 54, 12561.
doi: 10.1039/C8CC07434H |
|
|
(j) Ye, S.; Qiu, G.; Wu, J. Chem. Commun. 2019, 55, 1013.
doi: 10.1039/C8CC09250H |
|
|
(k) Ye, S.; Yang, M.; Wu, J. Chem. Commun. 2020, 56, 4145.
doi: 10.1039/D0CC01775B |
|
|
(l) Ye, S.; Li, X.; Xie, W.; Wu, J. Eur. J. Org. Chem. 2020, 2020, 1274.
doi: 10.1002/ejoc.201900396 |
|
|
(m) Xiao, W.; Wang, X.; Liu, R.; Wu, J. Chin. Chem. Lett. 2021, 32, 1847.
doi: 10.1016/j.cclet.2021.02.009 |
|
| [12] |
(a) Wang, X.; Xue, L.; Wang, Z. Org. Lett. 2014, 16, 4056.
doi: 10.1021/ol5018849 |
|
(b) Liu, N. W.; Liang, S.; Manolikakes, G. Adv. Synth. Catal. 2017, 359, 1308.
doi: 10.1002/adsc.v359.8 |
|
|
(c) Wang, H.; Sun, S.; Cheng, J. Org. Lett. 2017, 19, 5844.
doi: 10.1021/acs.orglett.7b02827 |
|
|
(d) Tang, N.; Shao, X.; Wang, M.; Wu, X.; Zhu, C. Acta Chim. Sinica 2019, 77, 922. (in Chinese)
doi: 10.6023/A19050158 |
|
|
( 汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨, 化学学报 2019, 77, 922)
|
|
|
(e) Sheng, J.; Li, Y.; Qiu, G. Org. Chem. Front. 2017, 4, 95.
doi: 10.1039/C6QO00530F |
|
|
(f) Wang, Y.; Deng, L.; Zhou, J. Adv. Synth. Catal. 2018, 360, 1060.
doi: 10.1002/adsc.v360.6 |
|
|
(g) Tribby, A. L.; Rodríguez, I.; Shariffudin, S.; Ball, N. D. J. Org. Chem. 2017, 82, 2294.
doi: 10.1021/acs.joc.7b00051 |
|
| [13] |
(a) Zheng, D.; Yu, J.; Wu, J. Angew. Chem. Int. Ed. 2016, 55, 11925.
doi: 10.1002/anie.201607292 |
|
(b) Zhang, J.; An, Y.; Wu, J. Chem. Eur. J. 2017, 23, 9477.
doi: 10.1002/chem.v23.40 |
|
|
(c) Liu, T.; Zheng, D.; Li, Z.; Wu, J. Adv. Synth. Catal. 2017, 359, 2653.
doi: 10.1002/adsc.v359.15 |
|
|
(d) An, Y.; Wu, J. Org. Lett. 2017, 19, 6028.
doi: 10.1021/acs.orglett.7b03195 |
|
|
(e) Liu, T.; Zheng, D.; Ding, Y.; Fan, X.; Wu, J. Chem. Asian J. 2017, 12, 465.
doi: 10.1002/asia.v12.4 |
|
|
(f) An, Y.; Zhang, J.; Xia, H.; Wu, J. Org. Chem. Front. 2017, 4, 1318.
doi: 10.1039/C7QO00193B |
|
|
(g) Wang, X.; Liu, T.; Zhong, Q.; Wu, J. Org. Chem. Front. 2017, 4, 2455.
doi: 10.1039/C7QO00787F |
|
|
(h) Liu, T.; Zheng, D.; Wu, J. Org. Chem. Front. 2017, 4, 1079.
doi: 10.1039/C7QO00075H |
|
|
(i) Zhang, J.; Zhou, K.; Wu, J. Org. Chem. Front. 2018, 5, 813.
doi: 10.1039/C7QO00987A |
|
|
(j) Liu, T.; Zheng, D.; Li, Z.; Wu, J. Adv. Synth. Catal. 2018, 360, 865.
doi: 10.1002/adsc.v360.5 |
|
|
(k) Zhang, J.; Zhang, F.; Lai, L. Chem. Commun. 2018, 54, 3891.
doi: 10.1039/C8CC01124A |
|
|
(l) Zhou, K.; Zhang, J.; Qiu, G.; Wu, J. Org. Lett. 2019, 21, 275.
doi: 10.1021/acs.orglett.8b03718 |
|
|
(m) Gong, X.; Li, X.; Xie, W.; Wu, J.; Ye, S. Org. Chem. Front. 2019, 6, 1863.
doi: 10.1039/C9QO00410F |
|
|
(n) Wang, X.; Lin, Y.; Liu, J. B. Chin. J. Chem. 2020, 38, 1098.
doi: 10.1002/cjoc.v38.10 |
|
|
(o) He, F. S.; Yao, Y.; Xie, W.; Wu, J. Chem. Commun. 2020, 56, 9469.
doi: 10.1039/D0CC03591B |
|
|
(p) Zhu, T.; Wu, J. Org. Lett. 2020, 22, 7094.
doi: 10.1021/acs.orglett.0c02400 |
|
|
(q) Huang, J.; Ding, F.; Chen, Z.; Yang, G.; Wu, J. Org. Chem. Front. 2021, 8, 1461.
doi: 10.1039/D0QO01546F |
|
|
(r) Yao, Y.; Yin, Z.; He, F. S.; Qin, X.; Xie, W.; Wu, J. Chem. Commun. 2021, 57, 2883.
doi: 10.1039/D0CC07927H |
|
| [14] |
(a) Zhou, K.; Huang, J.; Wu, J.; Qiu, G. Chin. Chem. Lett. 2021, 32, 37.
doi: 10.1016/j.cclet.2020.11.049 |
|
(b) Liu, T.; Ding, Y.; Fan, X.; Wu, J. Org. Chem. Front. 2018, 5, 3153.
doi: 10.1039/C8QO00965A |
|
|
(c) Ye, S.; Li, X.; Xie, W.; Wu, J. Asian J. Org. Chem. 2019, 8, 893.
doi: 10.1002/ajoc.v8.6 |
|
|
(d) Gong, X.; Yang, M.; Liu, J. B.; He, F. S.; Wu, J. Org. Chem. Front. 2020, 7, 938.
doi: 10.1039/D0QO00100G |
|
|
(e) Yang, M.; Han, H.; Jiang, H.; Ye, S.; Fan, X.; Wu, J. Chin. Chem. Lett. 2021, DOI: 10.1016j.cclet.2021.05.007.
|
|
| [15] |
(a) Wang, X.; Kuang, Y.; Ye, S.; Wu, J. Chem. Commun. 2019, 55, 14962
doi: 10.1039/C9CC08333B |
|
(b) Zhu, T.; Shen, J.; Sun, Y.; Wu, J. Chem. Commun. 2021, 57, 915.
doi: 10.1039/D0CC07632E |
|
| [16] |
(a) Wang, X.; Yang, M.; Xie, W.; Fan, X.; Wu, J. Chem. Commun. 2019, 55, 6010.
doi: 10.1039/C9CC03004B |
|
(b) Wang, X.; Li, H.; Qiu, G.; Wu, J. Chem. Commun. 2019, 55, 2062.
doi: 10.1039/C8CC10246E |
|
|
(c) Gong, X.; Yang, M.; Liu, J. B.; He, F.-S.; Fan, X.; Wu, J. Green Chem. 2020, 22, 1906.
doi: 10.1039/D0GC00332H |
|
| [17] |
(a) Zhao, Q.; Ren, L.; Hou, J.; Yu, W.; Chang, J. Org. Lett. 2019, 21, 210.
doi: 10.1021/acs.orglett.8b03663 |
|
(b) Huang, Z.; Zhang, Q.; Zhao, Q.; Yu, W.; Chang, J. Org. Lett. 2020, 22, 4378.
doi: 10.1021/acs.orglett.0c01393 |
|
| [18] |
Zhang, Q.-R.; Xue, D.-Q.; He, P.; Shao, K.-P.; Chen, P.-J.; Gu, Y.-F.; Ren, J.-L.; Shan, L.-H.; Liu, H.-M. Bioorg. Med. Chem. Lett. 2014, 24, 1236.
doi: 10.1016/j.bmcl.2013.12.010 |
| [19] |
Ito, S.; Tanaka, Y.; Kakehi, A. Bull. Chem. Soc. Jpn. 1984, 57, 539.
doi: 10.1246/bcsj.57.539 |
| [20] |
(a) Hossain, A.; Engl, S.; Lutsker, E.; Reiser, O. ACS Catal. 2019, 9, 1103.
doi: 10.1021/acscatal.8b04188 |
|
(b) Alkan-Zambada, M.; Hu, X. J. Org. Chem. 2019, 84, 4525.
doi: 10.1021/acs.joc.9b00238 |
|
| [21] |
Zhao, Q.; Ren, L.; Hou, J.; Yu, W.; Chang, J. Org. Lett. 2019, 21, 210.
doi: 10.1021/acs.orglett.8b03663 |
| [1] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [2] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [3] | 马智烨, 叶丽, 吴雨桓, 赵彤. B,N-SnO2/TiO2光催化剂的制备及其光催化性能研究[J]. 化学学报, 2021, 79(9): 1173-1179. |
| [4] | 董奎, 刘强, 吴骊珠. 放氢交叉偶联反应[J]. 化学学报, 2020, 78(4): 299-310. |
| [5] | 张振, 龚莉, 周晓渝, 颜思顺, 李静, 余达刚. 二氧化碳参与的自由基型烯烃双官能团化反应[J]. 化学学报, 2019, 77(9): 783-793. |
| [6] | 汤娜娜, 邵鑫, 王明扬, 吴新鑫, 朱晨. 磺酰氯参与的基于远端炔基迁移的非活化烯烃炔基化反应[J]. 化学学报, 2019, 77(9): 922-926. |
| [7] | 陈奕霖, 常亮, 左智伟. 可见光催化诱导的Smiles重排研究进展[J]. 化学学报, 2019, 77(9): 794-802. |
| [8] | 戴建玲, 雷文龙, 刘强. 可见光驱使铜盐催化芳香烃二氟烷基化反应[J]. 化学学报, 2019, 77(9): 911-915. |
| [9] | 杨俊航, 傅晓波, 卢增辉, 朱钢国. 可见光催化烯烃砜基化启动的远程醛基碳-氢键直接硫化反应[J]. 化学学报, 2019, 77(9): 901-905. |
| [10] | 刘茹雪, 何小燕, 牛力同, 吕柏霖, 余菲, 张哲, 杨志旺. 具有分级纳米结构的In2S3/CdIn2S4在可见光下催化苯甲胺的氧化偶联反应[J]. 化学学报, 2019, 77(7): 653-660. |
| [11] | 孟双艳, 杨红菊, 朱楠, 杨娇, 杨瑞瑞, 杨志旺. BiOCl-ov/坡缕石复合可见光催化剂的制备及其对醇类的选择性氧化研究[J]. 化学学报, 2019, 77(5): 461-468. |
| [12] | 马亚丽, 刘茹雪, 孟双艳, 牛力同, 杨志旺, 雷自强. 以UiO-66为前驱体的Fe-ZrO2的制备及其可见光降解性能研究[J]. 化学学报, 2019, 77(2): 153-159. |
| [13] | 孟双艳, 王明明, 吕柏霖, 薛群基, 杨志旺. Eu掺杂的ZnO/MIL-53(Fe)光催化剂的合成及其对醇类选择性氧化的催化性能研究[J]. 化学学报, 2019, 77(11): 1184-1193. |
| [14] | 张文强, 李秋艳, 杨馨雨, 马征, 王欢欢, 王晓军. 含苯并噻二唑结构单元的金属有机骨架在可见光催化需氧氧化反应中的应用[J]. 化学学报, 2017, 75(1): 80-85. |
| [15] | 钟建基, 孟庆元, 陈彬, 佟振合, 吴骊珠. 可见光催化的交叉偶联放氢反应[J]. 化学学报, 2017, 75(1): 34-40. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||