化学学报 ›› 2022, Vol. 80 ›› Issue (6): 788-796.DOI: 10.6023/A22010057 上一篇 下一篇
研究论文
张谭a,c, 余钟亮b,*( ), 余嘉祺b, 万慧凝b, 包成宇b, 涂文强b, 杨颂c,d
), 余嘉祺b, 万慧凝b, 包成宇b, 涂文强b, 杨颂c,d
投稿日期:2022-01-30
发布日期:2022-07-07
通讯作者:
余钟亮
基金资助:
Tan Zhanga,c, Zhongliang Yub( ), Jiaqi Yub, Huining Wanb, Chengyu Baob, Wenqiang Tub, Song Yangc,d
), Jiaqi Yub, Huining Wanb, Chengyu Baob, Wenqiang Tub, Song Yangc,d
Received:2022-01-30
Published:2022-07-07
Contact:
Zhongliang Yu
Supported by:文章分享

化学链合成氨是一种新型的、环境友好的低压合成氨技术, 其借助于载氮体的传递作用实现固氮与释氮制氨的循环, 已受到产学界的广泛关注. 构建高效、绿色的载氮体是化学链合成氨技术的关键. 本工作通过一步热解法制备了负载型钼基载氮体, 并对其释氮、固氮及稳定性进行了研究. 结果表明: 在释氮阶段, 当热解制备温度为450 ℃时, 钼基载氮体的氢化产氨速率为最大, 可达20000 μmol•g-1•h-1; 固氮过程中, 氢气的引入加快了钼基载氮体从氮气中补充晶格氮的速率, 实现了载氮体的高效再生; 历经12次循环后, 负载型钼基载氮体(制备温度为600 ℃)的产氨速率基本稳定在1500 μmol•g-1•h-1. 本研究探索了负载型钼基载氮体化学链合成氨的可行性, 结果可为新型过渡金属基载氮体的开发及其化学链合成氨研究提供理论基础.
张谭, 余钟亮, 余嘉祺, 万慧凝, 包成宇, 涂文强, 杨颂. 基于高性能负载型钼基载氮体的化学链合成氨性能研究[J]. 化学学报, 2022, 80(6): 788-796.
Tan Zhang, Zhongliang Yu, Jiaqi Yu, Huining Wan, Chengyu Bao, Wenqiang Tu, Song Yang. Chemical Looping Ammonia Synthesis with High Performance Supported Molybdenum-based Nitrogen Carrier[J]. Acta Chimica Sinica, 2022, 80(6): 788-796.
| 样品名称a | 比表面积/(m2•g-1) | 平均孔径/nm | 孔体积/(cm3•g-1) |
|---|---|---|---|
| 载体材料ZSM-5 | 338.7 | 2.5 | 0.09 |
| 热解后样品 | 134.2 | 3.7 | 0.03 |
| 12次循环后样品 | 159.8 | 2.9 | 0.02 |
| 样品名称a | 比表面积/(m2•g-1) | 平均孔径/nm | 孔体积/(cm3•g-1) |
|---|---|---|---|
| 载体材料ZSM-5 | 338.7 | 2.5 | 0.09 |
| 热解后样品 | 134.2 | 3.7 | 0.03 |
| 12次循环后样品 | 159.8 | 2.9 | 0.02 |
| 热解温度/℃ | wN/% | wC/% | wH/% | wS/% | wMo/% | 化学式 |
|---|---|---|---|---|---|---|
| 400 | 1.68 | 1.97 | 0.24 | 0 | 30.34 | Mo2N0.76H0.74C |
| 450 | 1.60 | 1.58 | 0.21 | 0 | 33.84 | Mo2N0.65H0.58C0.74 |
| 500 | 1.44 | 1.16 | 0.19 | 0 | 35.38 | Mo2N0.55H0.51C0.52 |
| 550 | 1.39 | 0.82 | 0.20 | 0 | 33.47 | Mo2N0.57H0.58C0.39 |
| 600 | 2.68 | 1.29 | 0.74 | 0 | 41.57 | Mo2N0.88H1.7C0.5 |
| 热解温度/℃ | wN/% | wC/% | wH/% | wS/% | wMo/% | 化学式 |
|---|---|---|---|---|---|---|
| 400 | 1.68 | 1.97 | 0.24 | 0 | 30.34 | Mo2N0.76H0.74C |
| 450 | 1.60 | 1.58 | 0.21 | 0 | 33.84 | Mo2N0.65H0.58C0.74 |
| 500 | 1.44 | 1.16 | 0.19 | 0 | 35.38 | Mo2N0.55H0.51C0.52 |
| 550 | 1.39 | 0.82 | 0.20 | 0 | 33.47 | Mo2N0.57H0.58C0.39 |
| 600 | 2.68 | 1.29 | 0.74 | 0 | 41.57 | Mo2N0.88H1.7C0.5 |
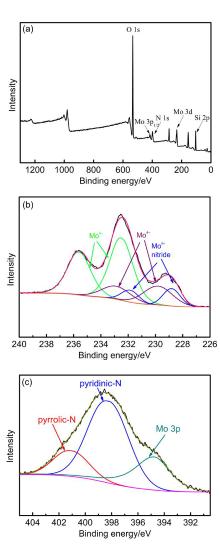


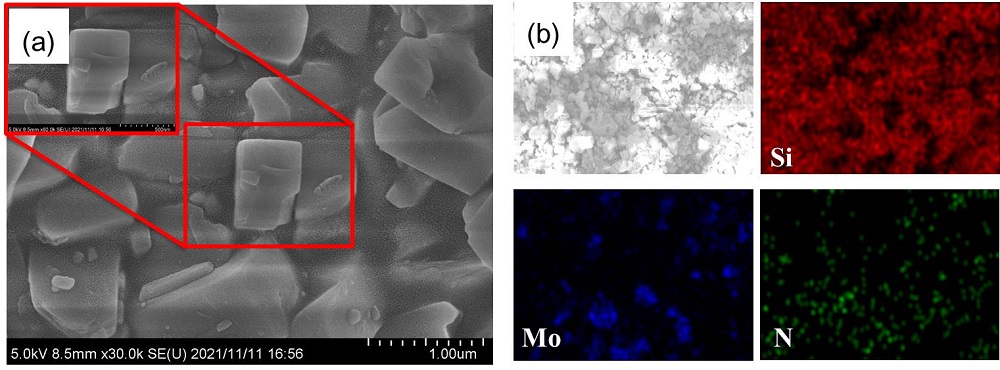
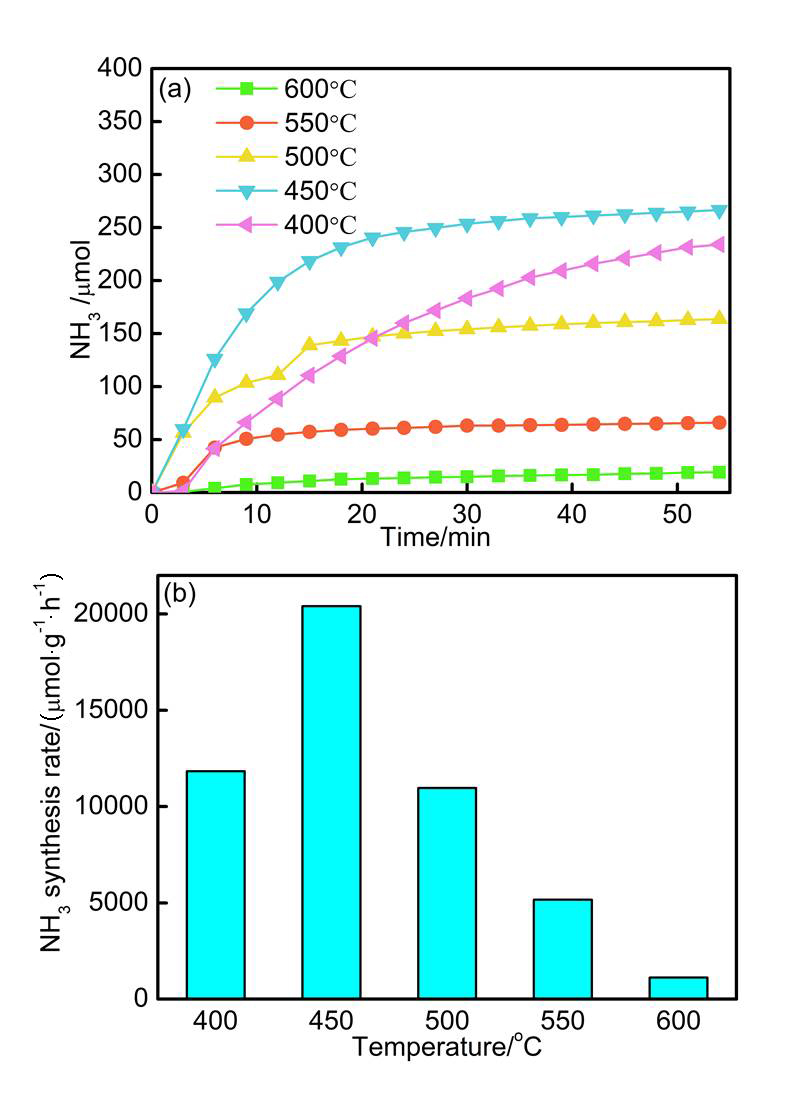
| No. | Metal nitrides | Condition | Ammonia production rate/ (μmol•g-1•h-1) | Ref. |
|---|---|---|---|---|
| 1 | Fe-Mn-N | 400 ℃ | 23 | [ |
| 2 | Co-Mn-N | 400 ℃ | 46 | [ |
| 3 | Fe3N | 400 ℃ | 50 | [ |
| 4 | Mn6N2.58 | 550 ℃ | 62 | [ |
| 5 | Ta3N5 | 400 ℃ | 80 | [ |
| 6 | Sr2N | 550 ℃ | 88 | [ |
| 7 | Ca3N2 | 550 ℃ | 121 | [ |
| 8 | Re3N | 350 ℃ | 130 | [ |
| 9 | Li-Mn-N | 400 ℃ | 261 | [ |
| 10 | Cu3N | 250 ℃ | 665 | [ |
| 11 | Zn3N2 | 400 ℃ | 852 | [ |
| 12 | Mo-N | 450 ℃ | 20000 | This work |
| No. | Metal nitrides | Condition | Ammonia production rate/ (μmol•g-1•h-1) | Ref. |
|---|---|---|---|---|
| 1 | Fe-Mn-N | 400 ℃ | 23 | [ |
| 2 | Co-Mn-N | 400 ℃ | 46 | [ |
| 3 | Fe3N | 400 ℃ | 50 | [ |
| 4 | Mn6N2.58 | 550 ℃ | 62 | [ |
| 5 | Ta3N5 | 400 ℃ | 80 | [ |
| 6 | Sr2N | 550 ℃ | 88 | [ |
| 7 | Ca3N2 | 550 ℃ | 121 | [ |
| 8 | Re3N | 350 ℃ | 130 | [ |
| 9 | Li-Mn-N | 400 ℃ | 261 | [ |
| 10 | Cu3N | 250 ℃ | 665 | [ |
| 11 | Zn3N2 | 400 ℃ | 852 | [ |
| 12 | Mo-N | 450 ℃ | 20000 | This work |
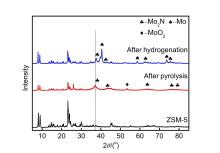
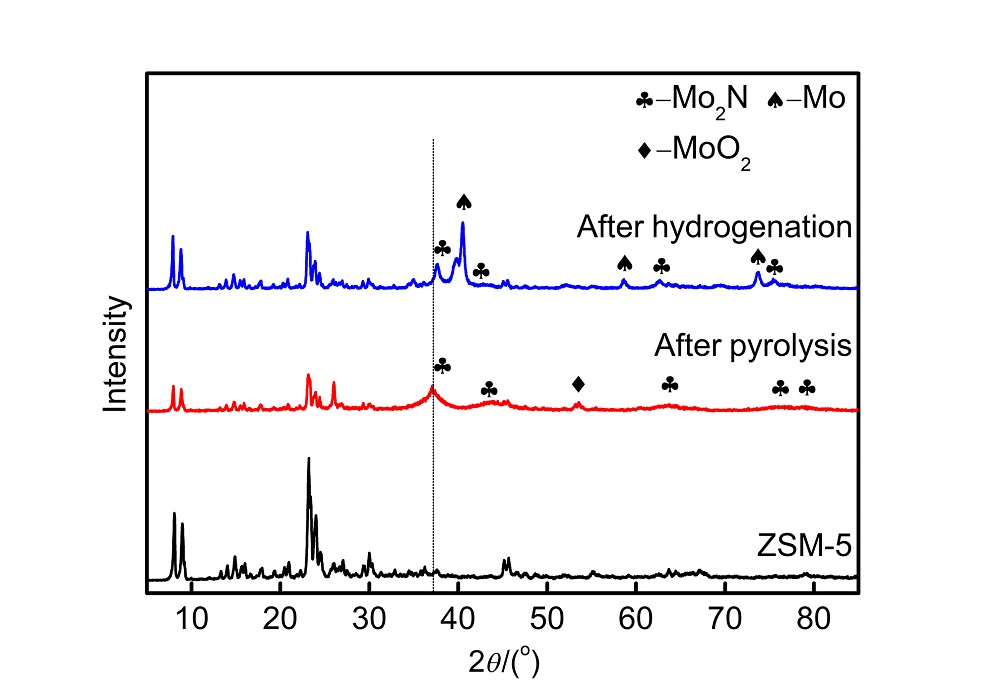
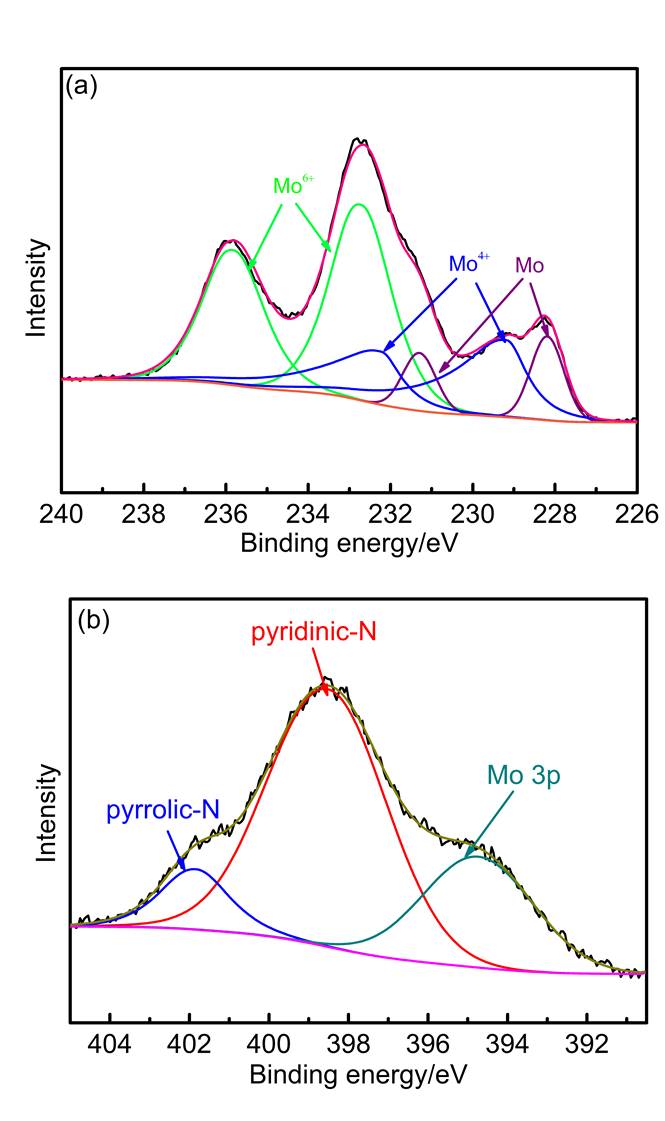




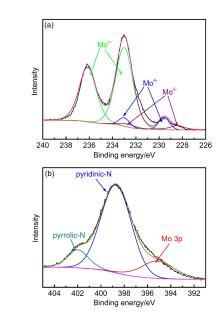

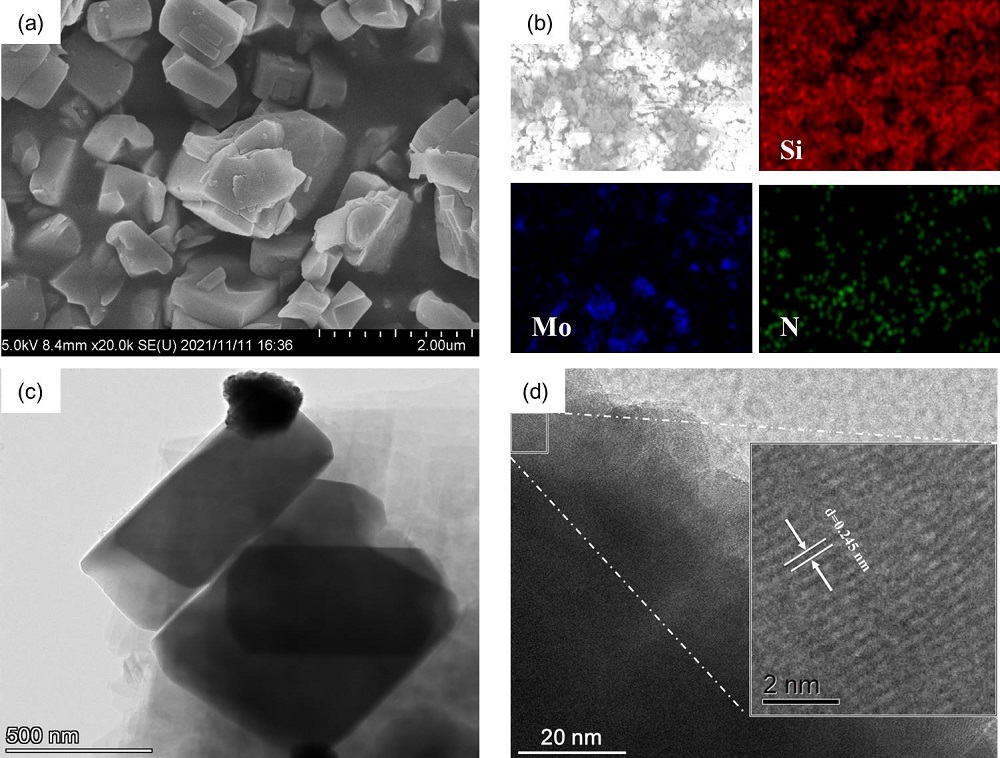
| 样品名称a | wN/% | wC/% | wH/% | wS/% |
|---|---|---|---|---|
| 热解后 | 2.67 | 1.29 | 0.74 | 0.00 |
| 氢化后 | 1.39 | 1.11 | 0.71 | 0.00 |
| 再生后 | 1.91 | 1.09 | 0.60 | 0.00 |
| 样品名称a | wN/% | wC/% | wH/% | wS/% |
|---|---|---|---|---|
| 热解后 | 2.67 | 1.29 | 0.74 | 0.00 |
| 氢化后 | 1.39 | 1.11 | 0.71 | 0.00 |
| 再生后 | 1.91 | 1.09 | 0.60 | 0.00 |


| [1] |
Klerke, A.; Christensen, C. H.; Norskov, J. K.; Vegge, T. J. Mater. Chem. 2008, 18, 2304.
doi: 10.1039/b720020j |
| [2] |
Guo, J. P.; Chen, P. Chem 2017, 3, 709.
doi: 10.1016/j.chempr.2017.10.004 |
| [3] |
Giddey, S.; Badwal, S. P. S.; Munnings, C.; Dolan, M. ACS Sustainable Chem. Eng. 2017, 5, 10231.
doi: 10.1021/acssuschemeng.7b02219 |
| [4] |
Liu, H. Chin. J. Catal. 2014, 35, 1619.
doi: 10.1016/S1872-2067(14)60118-2 |
| [5] |
Xu, T.; Ma, B.; Liang, J.; Yue, L.; Liu, Q.; Li, Y.; Zhao, H.; Luo, Y.; Lu, S.; Sun, X. Acta Phys.-Chim. Sin. 2021, 37, 109.
|
| [6] |
Wang, Q.; Guo, J.; Chen, P. J. Energy Chem. 2019, 36, 25.
doi: 10.1016/j.jechem.2019.01.027 |
| [7] |
Duan, Y.; Chen, C.; Zhang, J.; Wang, X.; Wei, J. Sci. Sin. Chim. 2020, 50, 337. (in Chinese)
doi: 10.1360/SSC-2019-0156 |
|
(段一菲, 陈存壮, 张军社, 王新赫, 魏进家, 中国科学: 化学, 2020, 50, 337.)
|
|
| [8] |
Guo, J.; Chen, P. Chin. Sci. Bull. 2019, 64, 1114 (in Chinses).
doi: 10.1360/N972019-00079 |
|
(郭建平, 陈萍, 科学通报, 2019, 64, 1114.)
|
|
| [9] |
Feng, S.; Gao, W.; Cao, H.; Guo, J.; Chen, P. Acta Chim. Sinica 2020, 78, 916. (in Chinese)
doi: 10.6023/A20060207 |
|
(冯圣, 高文波, 曹湖军, 陈建平, 陈萍, 化学学报, 2020, 78, 916.)
|
|
| [10] |
Feng, S.; Gao, W.; Wang, Q.; Guan, Y.; Yan, H.; Wu, H.; Cao, H.; Guo, J.; Chen, P. J. Mater. Chem. A 2021, 9, 1039.
doi: 10.1039/D0TA10519H |
| [11] |
Gao, W.; Guo, J.; Wang, P.; Wang, Q.; Chang, F.; Pei, Q.; Zhang, W.; Liu, L.; Chen, P. Nat. Energy. 2018, 3, 1067.
doi: 10.1038/s41560-018-0268-z |
| [12] |
Swearer, D. F.; Knowles, N. R.; Everitt, H. O.; Halas, N. J. ACS Energy Lett. 2019, 4, 1505.
doi: 10.1021/acsenergylett.9b00860 |
| [13] |
McEnaney, J. M.; Singh, A. R.; Schwalbe, J. A.; Kibsgaard, J.; Lin, J. C.; Cargnello, M.; Jaramillo, T. F.; Nørskov, J. K. Energy Environ. Sci. 2017, 10, 1621.
doi: 10.1039/C7EE01126A |
| [14] |
Kim, K.; Lee, S. J.; Kim, D. Y.; Yoo, C. Y.; Choi, J. W.; Kim, J. N.; Woo, Y.; Yoon, H. C.; Han, J. I. ChemSusChem 2018, 11, 120.
doi: 10.1002/cssc.201701975 |
| [15] |
Galvez, M. E.; Frei, A.; Halmann, M.; Steinfeld, A. Ind. Eng. Chem. Res. 2007, 46, 2047.
doi: 10.1021/ie061551m |
| [16] |
Feng, M.; Zhang, Q.; Wu, Y.; Liu, D. Energy Fuels 2020, 34, 12527.
doi: 10.1021/acs.energyfuels.0c02733 |
| [17] |
Gao, Y.; Wu, Y.; Zhang, Q.; Chen, X.; Jiang, G.; Liu, D. Int. J. Hydrog. Energy 2018, 43, 16589.
doi: 10.1016/j.ijhydene.2018.07.042 |
| [18] |
Wu, Y.; Gao, Y.; Zhang, Q.; Cai, T.; Chen, X.; Liu, D.; Fan, M. Fuel 2020, 264, 116821.
doi: 10.1016/j.fuel.2019.116821 |
| [19] |
Laassiri, S.; Zeinalipour-Yazdi, C. D.; Catlow, C. R. A.; Hargreaves, J. S. J. Appl. Catal., B 2018, 223, 60.
doi: 10.1016/j.apcatb.2017.04.073 |
| [20] |
Michalsky, R.; Parman, B. J.; Amanor-Boadu, V.; Pfromm, P. H. Energy 2012, 42, 251.
doi: 10.1016/j.energy.2012.03.062 |
| [21] |
Aframehr, W. M.; Huang, C.; Pfromm, P. H. Chem. Eng. Technol. 2020, 43, 2126.
doi: 10.1002/ceat.202000154 |
| [22] |
Hua, J. L.; Wang, K.; Wang, Q.; Peng, R. J. J. Therm. Anal. Calorim. 2021, 146, 673.
doi: 10.1007/s10973-020-10029-x |
| [23] |
He, Z.; Jiang, Y.; Cui, X.; Liu, Z.; Meng, X.; Wan, J.; Ma, F. ACS Appl. Nano Mater. 2022, 5, 5470.
doi: 10.1021/acsanm.2c00467 |
| [24] |
Thangudu, S.; Wu, C.-H.; Lee, C.-H.; Hwang, K. C. ACS Sustain. Chem. Eng. 2021, 9, 8748.
doi: 10.1021/acssuschemeng.1c01208 |
| [25] |
Wang, J.; Jiang, Z.; Peng, G.; Hoenig, E.; Yan, G.; Wang, M.; Liu, Y.; Du, X.; Liu, C. Adv. Sci. (Weinh). 2022, 9, e2104857.
|
| [26] |
Song, Y.; Wang, H.; Song, Z.; Zheng, X.; Fan, B.; Han, X.; Deng, Y.; Hu, W. ACS Appl. Mater. Interfaces 2022, 14, 17273.
doi: 10.1021/acsami.2c00280 |
| [27] |
Huang, X.; Ding, X.; Wang, J.; Wang, Y.; Gurti, J. I.; Chen, Y.; Wang, M.; Li, W.; Wang, X. Struct. Chem. 2022, doi: 10.1007/s11224-022-01919-x
doi: 10.1007/s11224-022-01919-x |
| [28] |
Xu, K.; Feng, J.; Chu, Q.; Zhang, L.; Li, W. Acta Phys.-Chim. Sin. 2014, 30, 2063. (in Chinese)
doi: 10.3866/PKU.WHXB201409221 |
|
(徐坤, 冯杰, 褚绮, 张丽丽, 李文英, 物理化学学报, 2014, 30, 2063.)
|
|
| [29] |
Ren, X.; Cui, G.; Chen, L.; Xie, F.; Wei, Q.; Tian, Z.; Sun, X. Chem. Commun. 2018, 54, 8474.
doi: 10.1039/C8CC03627F |
| [30] |
Liu, N.; Nie, L.; Xue, N.; Dong, H.; Peng, L.; Guo, X.; Ding, W. ChemCatChem 2010, 2, 167.
doi: 10.1002/cctc.200900155 |
| [31] |
Ding, W.; Li, S.; Meitzner, G.; Iglesia, E. J. Phys. Chem. B 2001, 105, 506.
doi: 10.1021/jp0030692 |
| [32] |
Afanasiev, P. Inorg. Chem. 2002, 41, 5317.
pmid: 12377022 |
| [33] |
Yu, Z.; Yoshida, A.; Shi, J.; Wang, T.; Yang, S.; Ye, Q.; Hao, X.; Abudula, A.; Fang, Y.; Guan, G. ACS Sustain. Chem. Eng. 2020, 8, 13956.
doi: 10.1021/acssuschemeng.0c03269 |
| [34] |
Yu, Z.; An, X.; Kurnia, I.; Yoshida, A.; Yang, Y.; Hao, X.; Abudula, A.; Fang, Y.; Guan, G. ACS Catal. 2020, 10, 5353.
doi: 10.1021/acscatal.0c00752 |
| [35] |
Wang, B.; Guo, H.; Yin, X.; Shen, L. Energy Fuels 2020, 34, 10247.
doi: 10.1021/acs.energyfuels.0c01000 |
| [36] |
Wei, Z. B. Z.; Grange, P.; Delmon, B. Appl. Surf. Sci. 1998, 135, 107.
doi: 10.1016/S0169-4332(98)00267-0 |
| [37] |
Song, Y.; Yuan, Z. Electrochim. Acta 2017, 246, 536.
doi: 10.1016/j.electacta.2017.06.086 |
| [38] |
Roy, P. K.; Kumar, S. ACS Appl. Energy Mater. 2020, 3, 7167.
doi: 10.1021/acsaem.0c01209 |
| [39] |
Hargreaves, J. S. J.; McKay, D. J. Mol. Catal. A: Chem. 2009, 305, 125.
doi: 10.1016/j.molcata.2008.08.006 |
| [40] |
McKay, D.; Hargreaves, J. S. J.; Rico, J. L.; Rivera, J. L.; Sun, X. L. J. Solid State Chem. 2008, 181, 325.
doi: 10.1016/j.jssc.2007.12.001 |
| [41] |
Al Sobhi, S.; Bion, N.; Hargreaves, J. S. J.; Hector, A. L.; Laassiri, S.; Levason, W.; Lodge, A. W.; McFarlane, A. R.; Ritter, C. Mater. Res. Bull. 2019, 118, 110519.
doi: 10.1016/j.materresbull.2019.110519 |
| [42] |
Yang, S.; Zhang, T.; Yang, Y.; Wang, B.; Li, J.; Gong, Z.; Yao, Z.; Du, W.; Liu, S.; Yu, Z. Appl. Catal. B 2022, 312, 121404.
doi: 10.1016/j.apcatb.2022.121404 |
| [43] |
Alexander, A. M.; Hargreaves, J. S. J.; Mitchell, C. Top. Catal. 2013, 56, 1963.
doi: 10.1007/s11244-013-0133-z |
| [44] |
Michalsky, R.; Avram, A. M.; Peterson, B. A.; Pfromm, P. H.; Peterson, A. A. Chem. Sci. 2015, 6, 3965.
doi: 10.1039/c5sc00789e pmid: 29218166 |
| [45] |
Alexander, A. M.; Hargreaves, J. S. J.; Mitchell, C. Top. Catal. 2012, 55, 1046.
doi: 10.1007/s11244-012-9890-3 |
| [46] |
Gong, S.; Chen, H.; Li, W.; Li, B. Appl. Catal. A: Gen. 2005, 279, 257.
doi: 10.1016/j.apcata.2004.10.038 |
| [47] |
Michalsky, R.; Pfromm, P. H.; Steinfeld, A. Interface Focus. 2015, 5, 20140084.
doi: 10.1098/rsfs.2014.0084 |
| [48] |
Michalsky, R.; Pfromm, P. H. J. Phys. Chem. C 2012, 116, 23243.
doi: 10.1021/jp307382r |
| [49] |
Gregory, D. H.; Hargreaves, J. S. J.; Hunter, S. M. Catal. Lett. 2010, 141, 22.
doi: 10.1007/s10562-010-0464-3 |
| [50] |
McKay, D.; Gregory, D. H.; Hargreaves, J. S.; Hunter, S. M.; Sun, X. Chem. Commun. 2007, 3051.
|
| [51] |
Sato, K.; Imamura, K.; Kawano, Y.; Miyahara, S.; Yamamoto, T.; Matsumura, S.; Nagaoka, K. Chem. Sci. 2017, 8, 674.
doi: 10.1039/C6SC02382G |
| [52] |
Kojima, R.; Aika, K. Appl. Catal. A-Gen. 2001, 219, 141.
doi: 10.1016/S0926-860X(01)00676-7 |
| [1] | 陈霄, 许汉华, 石向辉, 魏俊年, 席振峰. 稀土和锕系配合物促进的氮气活化与转化研究[J]. 化学学报, 2022, 80(9): 1299-1308. |
| [2] | 马行宇, 孙晖, 李江, 刘之洋, 周红军. 基于Li-N2电池体系的“连续式”氮气还原合成氨[J]. 化学学报, 2022, 80(7): 861-866. |
| [3] | 李嘉鹏, 殷剑昊, 俞超, 张文雄, 席振峰. 从氮气直接合成含氮有机化合物[J]. 化学学报, 2017, 75(8): 733-743. |
| [4] | 戴立信,陈耀全. 创造更美好的生活和更清洁的环境: 化学的回顾与展望[J]. 化学学报, 2000, 58(1): 1-5. |
| [5] | 陈忠,林国兴,蔡淑惠,徐昕,黄静伟,万惠霖,蔡启瑞. 重水中固氮酶催化还原乙炔产物的^1H NMR研究[J]. 化学学报, 1999, 57(8): 907-913. |
| [6] | 康北笙,蔡进华,吴达旭,刘汉钦,刘秋田,翁林红,卢嘉锡. 铁铂硫原子簇化合物的合成与结构研究 V: [MoFe3S4]类立方烷簇合物的转化反应和[Et4N]3[Mo2Fe6S8(SC6H4CH3-m)3Cl6]的晶体结构[J]. 化学学报, 1989, 47(8): 744-750. |
| [7] | 余秀芬,杨融生,李俊. 若干含氧桥双核钼(V)原子簇化合物的研究[J]. 化学学报, 1989, 47(8): 738-743. |
| [8] | 杨光第,孙宏林,徐吉庆,千吉松,魏诠. [(C2H5)4N]2{Fe4S4[S2CN(C2H5)2]4}的晶体和分子结构[J]. 化学学报, 1989, 47(1): 1-5. |
| [9] | 庄伯涛,黄梁仁,何玲洁,杨瑜,卢嘉锡. 含SR桥的双核钼(I)配合物Mo2(CO)8-n(SR)2Ln的新合成途径和Mo2(C(O)6(SPh)2(MeCN)2的结构研究[J]. 化学学报, 1989, 47(1): 25-30. |
| [10] | 刘春万,华建民,卢嘉锡. 固氮酶底物配位活化的量子化学模拟[J]. 化学学报, 1988, 46(4): 315-320. |
| [11] | 刘秋田,黄梁仁,杨瑜,卢嘉锡. MoFe3S4单立方烷原子簇研究 IV:新型的双跨桥六配体单立方烷簇合物MoFe3S4(μ-Me2NCS2)2(Me2NCS2)4.2CH3CN的合成与结构[J]. 化学学报, 1988, 46(11): 1075-1080. |
| [12] | 刘平,黄梁仁,王玲玲,康北笙,卢嘉锡. 含Fe3S3簇核的两个异构体化合物的合成和结构研究[J]. 化学学报, 1988, 46(10): 972-977. |
| [13] | 刘秋田,黄梁仁,杨瑜,卢嘉锡. MoFe3S4单立方烷原子簇研究 III: 三核链状簇合物向单立方烷簇合物的转化及MoFe3S4(Et2NCS2)5.CH3CN的结构[J]. 化学学报, 1988, 46(1): 1-8. |
| [14] | 何玲洁,张琳娜,卢嘉锡. 若干线型Mo-Fe-S簇合物红外光谱及其与结构关系的研究[J]. 化学学报, 1987, 45(7): 676-681. |
| [15] | 林振阳,刘春万. Fe-S簇合物的Fe-Fe相互作用及电子结构规律[J]. 化学学报, 1987, 45(6): 535-540. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||