化学学报 ›› 2023, Vol. 81 ›› Issue (1): 64-83.DOI: 10.6023/A22100422 上一篇 下一篇
综述
王晓晨a, 季泽尧a, 刘健a, 王炳福a,b, 金辉a,*( ), 张立新a,*(
), 张立新a,*( )
)
投稿日期:2022-10-10
发布日期:2022-12-05
作者简介: |
王晓晨, 1994年出生于山东菏泽, 2018年在山东第一医科大学获得学士学位, 2020年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展以硫酯为底物的合成方法学合成具有潜在生物活性的分子. |
 |
季泽尧, 1998年出生于河南南阳, 2021年毕业于吉林化工学院制药工程专业获得学士学位. 2021年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
刘健, 1996年出生于辽宁鞍山, 2019年在辽宁科技大学获得学士学位. 2021年至今在张立新教授和金辉博士的指导下攻读硕士学位. 研究兴趣是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
王炳福, 1992年出生于黑龙江哈尔滨, 2015年和2018年在辽宁科技大学先后获得学士和硕士学位, 2019年至今, 在张立新教授和金辉博士的指导下攻读博士学位. 研究方向为不对称有机小分子催化. |
 |
金辉讲师、硕士生导师. 1989年出生于辽宁丹东, 2011年在大连医科大学获得学士学位(药学). 2014年在沈阳药科大学获得硕士学位(药物化学). 2014年至2018年在韩国成均馆大学化学系学习并获得有机化学博士学位(导师:Do Hyun Ryu教授). 2018年加入沈阳化工大学功能分子研究所从事科研教学工作. 研究兴趣主要是发展新的合成方法学合成具有潜在生物活性的分子. |
 |
张立新教授、博士生导师. 1966年出生于辽宁锦州, 1987年在兰州大学获得学士学位. 1993年在沈阳化工研究院获得硕士学位. 2000年至2004年在英国利兹大学化学系学习并获得有机化学博士学位(导师:Ronald Grigg教授). 曾就职于沈阳化工研究院(1987~2000, 高级工程师)、2016年加入沈阳化工大学, 组建功能分子研究所. 研究兴趣主要是新农药、医药创制和有机合成方法学. |
基金资助:
Xiaochen Wanga, Zeyao Jia, Jian Liua, Bingfu Wanga,b, Hui Jina( ), Lixin Zhanga(
), Lixin Zhanga( )
)
Received:2022-10-10
Published:2022-12-05
Contact:
*E-mail: Supported by:文章分享
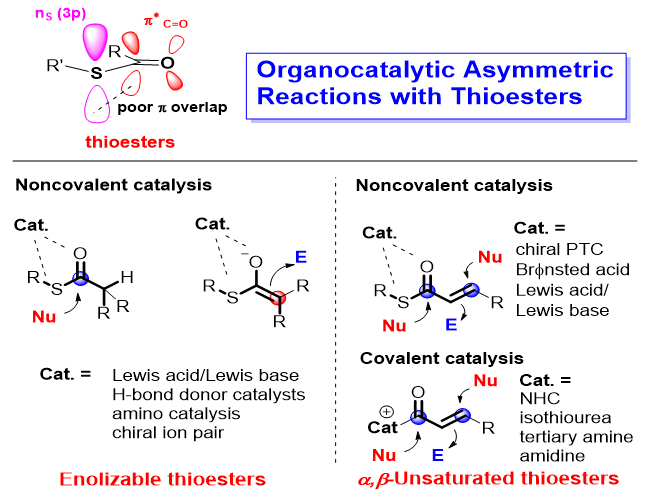
硫酯在生物合成和有机合成中扮演着十分重要的角色. 硫酯的羰基C(2p)轨道和S(3p)轨道重叠度较小, 其α质子具有较强的酸性, 能在温和的条件下烯醇化并进行亲核反应. 同时, 硫酯还是高效的酰基化试剂, 可用于酯键和酰胺键的构建. 有机小分子催化是不对称催化的重要研究领域. 近十几年来有机催化硫酯底物的不对称反应取得了大量重要成果, 极大拓宽了有机催化酯类底物的反应类型, 并实现了一些其氧酯类似物无法实现的反应. 本综述按照硫酯底物的类型(可烯醇化硫酯和α,β-不饱和硫酯)、有机催化剂类型和反应类型对硫酯底物参与的有机催化不对称反应进行梳理和总结, 同时对代表性反应的机理以及该领域的未来发展进行了简单阐述.
王晓晨, 季泽尧, 刘健, 王炳福, 金辉, 张立新. 硫酯参与的有机催化不对称反应研究进展[J]. 化学学报, 2023, 81(1): 64-83.
Xiaochen Wang, Zeyao Ji, Jian Liu, Bingfu Wang, Hui Jin, Lixin Zhang. Advances in Organocatalytic Asymmetric Reactions Involving Thioesters[J]. Acta Chimica Sinica, 2023, 81(1): 64-83.
| 序号 | 标题 |
|---|---|
| 1 | 引言 |
| 2 | 可烯醇化硫酯参与的不对称反应 |
| 2.1 | 多氟烷基取代硫酯参与的反应 |
| 2.2 | α-羰基取代硫酯参与的反应 |
| 2.2.1 | 丙二酸半硫酯参与的反应 |
| 2.2.2 | 丙二酸单硫酯参与的反应 |
| 2.2.3 | 丙二酸二硫酯和β-酮硫酯参与的反应 |
| 2.2.4 | α-氮取代乙酸硫酯参与的反应 |
| 3 | α,β-不饱和硫酯参与的不对称反应 |
| 3.1 | 非共价催化α,β-不饱和硫酯的反应 |
| 3.2 | 共价催化α,β-不饱和硫酯的反应 |
| 4 | 总结与展望 |
| 序号 | 标题 |
|---|---|
| 1 | 引言 |
| 2 | 可烯醇化硫酯参与的不对称反应 |
| 2.1 | 多氟烷基取代硫酯参与的反应 |
| 2.2 | α-羰基取代硫酯参与的反应 |
| 2.2.1 | 丙二酸半硫酯参与的反应 |
| 2.2.2 | 丙二酸单硫酯参与的反应 |
| 2.2.3 | 丙二酸二硫酯和β-酮硫酯参与的反应 |
| 2.2.4 | α-氮取代乙酸硫酯参与的反应 |
| 3 | α,β-不饱和硫酯参与的不对称反应 |
| 3.1 | 非共价催化α,β-不饱和硫酯的反应 |
| 3.2 | 共价催化α,β-不饱和硫酯的反应 |
| 4 | 总结与展望 |
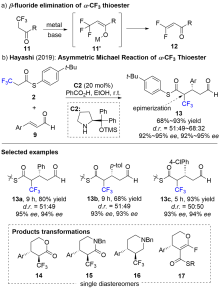

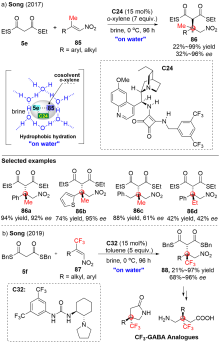

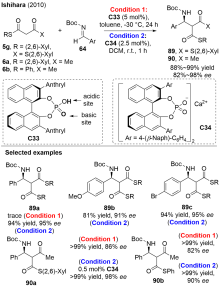


| [1] |
(a) Ding, K. L.; Fan, Q. H. Asymmetric Catalysis: New Concepts and Methods, Chemical Industry Press, Beijing, 2009. (in Chinese)
|
|
( 丁奎岭, 范青华, 不对称催化新概念与新方法, 化学工业出版社, 北京, 2009.)
|
|
|
(b) Ding, K. L. Acta Chim. Sinica 2014, 72, 755. (in Chinese)
doi: 10.6023/A1407E001 |
|
|
( 丁奎岭, 化学学报 2014, 72, 755.)
doi: 10.6023/A1407E001 |
|
|
(c) Dalko, P. I. Comprehensive Enantioselective Organocatalysis: Catalysts, Reactions, and Applications, Wiley-VCH Verlag GmbH, Weinheim, 2013.
|
|
| [2] |
Saha, D.; Kharbanda, A.; Yan, W.; Lakkaniga, N. R.; Frett, B.; Li, H.-y. J. Med. Chem. 2020, 63, 441.
doi: 10.1021/acs.jmedchem.9b00640 |
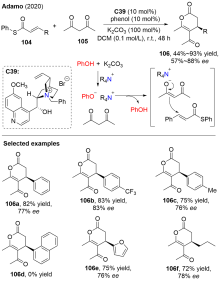
| [3] |
(a) Mulholland, A. J.; Lyne, P. D.; Karplus, M. J. Am. Chem. Soc. 2000, 122, 534.
doi: 10.1021/ja992874v pmid: 8011640 |
|
(b) Usher, K. C.; Remington, S. J.; Martin, D. P.; Drueckhammer, D. G. Biochemistry 1994, 33, 7753.
pmid: 8011640 |
|
|
(c) Wiegand, G.; Remington, S. J. Ann. Rev. Biophys. Chem. 1986, 15, 97.
pmid: 8011640 |
|
| [4] |
(a) Hill, A. M. Nat. Prod. Rep. 2006, 23, 256.
doi: 10.1039/B301028G pmid: 11548049 |
|
(b) Staunton, J.; Weissman, K. J. Nat. Prod. Rep. 2001, 18, 380.
doi: 10.1039/a909079g pmid: 11548049 |
|
| [5] |
(a) Masamune, S.; Kamata, S.; Schilling, W. J. Am. Chem. Soc. 1975, 97, 3515.
pmid: 893906 |
|
(b) Masamune, S.; Hayase, Y.; Schilling, W.; Chan, W. K.; Bates, G. S. J. Am. Chem. Soc. 1977, 99, 6756.
pmid: 893906 |
|
| [6] |
Yang, X.; Ma, Y.; Di, H.; Wang, X.; Jin, H.; Ryu, D. H.; Zhang, L. Adv. Synth. Catal. 2021, 363, 3201.
doi: 10.1002/adsc.202100369 |
| [7] |
(a) Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Fukuyama, T. Tetrahedron Lett. 1998, 39, 3189.
|
|
(b) Miyazaki, T.; Han-ya, Y.; Tokuyama, H.; Fukuyama, T. Synlett 2004, 477.
|
|
|
(c) Fukuyama, T.; Tokuyama, H. Aldrichimica Acta 2004, 37, 87.
|
|
|
(d) Mori, Y.; Seki, M. Adv. Synth. Catal. 2007, 349, 2027.
doi: 10.1002/adsc.200600610 |
|
|
(e) Cherney, A. H.; Reisman, S. E. Tetrahedron 2014, 70, 3259.
doi: 10.1016/j.tet.2013.11.104 |
|
| [8] |
Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5607.
|
| [9] |
(a) Hirschbeck, V.; Gehrtz, P. H.; Fleischer, I. Chem. Eur. J. 2018, 24, 7092.
doi: 10.1002/chem.201705025 |
|
(b) Ye, Q.; Cao, W.-G.; Gao, J.-S. Chin. J. Org. Chem. 2002, 21, 697. (in Chinese)
|
|
|
( 叶青, 曹卫国, 高金森, 有机化学, 2002, 21, 697.)
|
|
| [10] |
(a) Magdziak, D.; Lalic, G.; Lee, M. H.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284.
pmid: 15898756 |
|
(b) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747.
pmid: 15898756 |
|
|
(c) Sawa, M.; Miyazaki, S.; Yonesaki, R.; Morimoto, H.; Ohshima, T. Org. Lett. 2018, 20, 5393.
doi: 10.1021/acs.orglett.8b02306 pmid: 15898756 |
|
|
(d) Furutachi, M.; Mouri, S.; Matsunaga, S.; Shibasaki, M. Chem. Asian J. 2010, 5, 2351.
doi: 10.1002/asia.201000540 pmid: 15898756 |
|
| [11] |
Walker, M. C.; Thuronyi, B. W.; Charkoudian, L. K.; Lowry, B.; Khosla, C.; Chang, M. C. Y. Science 2013, 341, 1089.
doi: 10.1126/science.1242345 pmid: 24009388 |
| [12] |
(a) Xiang, S.-H.; Tan, B. Nat. Commun. 2020, 11, 3786.
doi: 10.1038/s41467-020-17580-z |
|
(b) Zhang, M.-M.; Luo, Y.-Y.; Lu, L.-Q.; Xiao, W.-J. Acta Chim. Sinica 2018, 76, 838. (in Chinese)
doi: 10.6023/A18060237 |
|
|
( 张毛毛, 骆元元, 陆良秋, 肖文精, 化学学报 2018, 76, 838.)
doi: 10.6023/A18060237 |
|
|
(c) Di, H.; Liu, Y.; Ma, Y.; Yang, X.; Jin, H.; Zhang, L. Chin. J. Org. Chem. 2021, 41, 2228. (in Chinese)
doi: 10.6023/cjoc202010039 |
|
|
( 底慧明, 刘云婷, 马艳榕, 杨鑫悦, 金辉, 张立新, 有机化学, 2021, 41, 2228.)
doi: 10.6023/cjoc202010039 |
|
|
(d) Ren, H.; Ma, M.; Huang, Y. Chin. J. Org. Chem. 2022, 42, 3129. (in Chinese)
doi: 10.6023/cjoc202208024 |
|
|
任红霞, 马萌萌, 黄有, 有机化学, 2022, 42, 3129.).
doi: 10.6023/cjoc202208024 |
|
|
(e) Chen, X.; Wang, H.; Jin, Z.; Chi, Y. R. Chin. J. Chem. 2020, 38, 1167.
doi: 10.1002/cjoc.202000107 |
|
| [13] |
Bordwell, F. G.; Fried, H. E. J. Org. Chem. 1991, 56, 4218.
doi: 10.1021/jo00013a027 |
| [14] |
Um, P.-J.; Drueckhammer, D. G. J. Am. Chem. Soc. 1998, 120, 5605.
doi: 10.1021/ja980445b |
| [15] |
Alonso, D. A.; Kitagaki, S.; Utsumi, N.; Barbas III, C. F. Angew. Chem., Int. Ed. 2008, 47, 4588.
doi: 10.1002/anie.200801088 pmid: 18464230 |
| [16] |
Hayashi, Y.; Yamada, T.; Sato, M.; Watanabe, S.; Kwon, E.; Iwasaki, K.; Umemiya, S. Org. Lett. 2019, 21, 5183.
doi: 10.1021/acs.orglett.9b01774 pmid: 31247799 |
| [17] |
Kohler, M. C.; Yost, J. M.; Garnsey, M. R.; Coltart, D. M. Org. Lett. 2010, 12, 3376.
doi: 10.1021/ol101152b pmid: 20608684 |
| [18] |
Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed., Oxford University Press, New York, 2012, pp. 628-630.
|
| [19] |
Kobuke, Y.; Yoshida, J.-i. Tetrahedron Lett. 1978, 19, 367.
doi: 10.1016/S0040-4039(01)85127-3 |
| [20] |
(a) Magdziak, D.; Lalic, G.; Lee, H. M.; Fortner, K. C.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2005, 127, 7284.
pmid: 17263375 |
|
(b) Fortner, K. C.; Shair, M. D. J. Am. Chem. Soc. 2007, 129, 1032.
pmid: 17263375 |
|
|
(c) Lalic, G.; Aloise, A. D.; Shair, M. D. J. Am. Chem. Soc. 2003, 125, 2852.
doi: 10.1021/ja029452x pmid: 17263375 |
|
|
(d) Orlandi, S.; Benaglia, M.; Cozzi, F. Tetrahedron Lett. 2004, 45, 1747.
pmid: 17263375 |
|
| [21] |
Bae, H. Y.; Sim, J. H.; Lee, J.-W.; List, B.; Song, C. E. Angew. Chem., Int. Ed. 2013, 52, 12143.
doi: 10.1002/anie.201306297 |
| [22] |
Wang, Y.; Huang, G.; Hu, S.; Jin, K.; Wu, Y.; Chen, F. Tetrahedron Lett. 2017, 73, 5055.
doi: 10.1016/j.tet.2017.05.066 |
| [23] |
(a) Müller, K.; Faeh, C.; Diederich, F. Science 2007, 317, 1881.
doi: 10.1126/science.1131943 pmid: 18197347 |
|
(b) Purser, S.; Moore, P. R.; Swallow, S.; Gouverneur, V. Chem. Soc. Rev. 2008, 37, 32.
pmid: 18197347 |
|
|
(c) O'Hagan, D. Chem. Soc. Rev. 2008, 37, 308.
doi: 10.1039/b711844a pmid: 18197347 |
|
| [24] |
Saadi, J.; Akakura, M.; Yamamoto, H. J. Am. Chem. Soc. 2011, 133, 14248.
doi: 10.1021/ja2066169 |
| [25] |
Saadi, J.; Wennemers, H. Nat. Chem. 2016, 8, 276.
doi: 10.1038/nchem.2437 |
| [26] |
Zetschok, D.; Heieck, L.; Wennemers, H. Org. Lett. 2021, 23, 1753.
doi: 10.1021/acs.orglett.1c00172 pmid: 33591192 |
| [27] |
(a) Bao, Y.; Kumagai, N.; Shibasaki, M. Chem. Sci. 2015, 6, 6124.
doi: 10.1039/C5SC02218E pmid: 19722664 |
|
(b) Misaki, T.; Takimoto, G.; Sugimura, T. J. Am. Chem. Soc. 2010, 132, 6286.
doi: 10.1021/ja101216x pmid: 19722664 |
|
|
(c) Yamaguchi, A.; Matsunaga, S.; Shibasaki, M. J. Am. Chem. Soc. 2009, 131, 10842.
doi: 10.1021/ja904575e pmid: 19722664 |
|
|
(d) Saito, S.; Kobayashi, S. J. Am. Chem. Soc. 2006, 128, 8704.
doi: 10.1021/ja061221t pmid: 19722664 |
|
|
(e) Suto, Y.; Tsuji, R.; Kanai, M.; Shibasaki, M. Org. Lett. 2005, 7, 3757.
doi: 10.1021/ol051423e pmid: 19722664 |
|
| [28] |
Park, J. H.; Sim, J. H.; Song, C. E. Org. Lett. 2019, 21, 4567.
doi: 10.1021/acs.orglett.9b01469 |
| [29] |
Ricci, A.; Pettersen, D.; Bernardi, L.; Fini, F.; Fochi, F.; Herrera, R. P.; Sgarzania, V. Adv. Synth. Catal. 2007, 349, 1037.
doi: 10.1002/adsc.200600536 |
| [30] |
Pan, Y. H.; Kee, C. W.; Jiang, Z. Y.; Ma, T.; Zhao, Y. J.; Yang, Y. Y.; Xue, H. S.; Tan, C. H. Chem. Eur. J. 2011, 17, 8363.
doi: 10.1002/chem.201100687 |
| [31] |
Hara, N.; Nakamura, S.; Sano, M. Chem. Eur. J. 2012, 18, 9276.
doi: 10.1002/chem.201200367 |
| [32] |
(a) Takashina, N.; Price, C. C. J. Am. Chem. Soc. 1962, 84, 489.
doi: 10.1021/ja00862a034 |
|
(b) Rauhut, M. M.; Currier, H. A.; Semsel, A. M.; Wystrach, V. P. J. Org. Chem. 1961, 26, 5138.
doi: 10.1021/jo01070a087 |
|
|
(c) Morita, K.-i.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815.
doi: 10.1246/bcsj.41.2815 |
|
| [33] |
(a) Lu, X.; Zhang, C.; Xu, Z. Acc. Chem. Res. 2001, 34, 535.
doi: 10.1021/ar000253x |
|
(b) Methot, J. L.; Roush, W. R. Adv. Synth. Catal. 2004, 346, 1035.
doi: 10.1002/adsc.200404087 |
|
|
(c) Lu, X.; Du, Y.; Lu, C. Pure Appl. Chem. 2005, 77, 1985.
doi: 10.1351/pac200577121985 |
|
|
(d) Ye, L.-W.; Zhou, J.; Tang, Y. Chem. Soc. Rev. 2008, 37, 1140.
doi: 10.1039/b717758e |
|
|
(e) Cowen, B. J.; Miller, S. J. Chem. Soc. Rev. 2009, 38, 3102.
doi: 10.1039/b816700c |
|
|
(f) Wei, Y.; Shi, M. Acc. Chem. Res. 2010, 43, 1005.
doi: 10.1021/ar900271g |
|
|
(g) Marinetti, A.; Voituriez, A. Synlett 2010, 2010, 174.
doi: 10.1055/s-0029-1219157 |
|
|
(h) Lu, Y.; Wang, S.-X.; Han, X.; Zhong, F.; Wang, Y. Synlett 2011, 2011, 2766.
doi: 10.1055/s-0031-1289538 |
|
|
(i) Zhao, Q.-Y.; Lian, Z.; Wei, Y.; Shi, M. Chem. Commun. 2012, 48, 1724.
doi: 10.1039/C1CC15793K |
|
|
(j) Fan, Y. C.; Kwon, O. Chem. Commun. 2013, 49, 11588.
doi: 10.1039/c3cc47368f |
|
|
(k) Wang, Z.; Xu, X.; Kwon, O. Chem. Soc. Rev. 2014, 43, 2927.
doi: 10.1039/C4CS00054D |
|
|
(l) Xiao, Y.; Sun, Z.; Guo, H.; Kwon, O. J. Org. Chem. 2014, 10, 2089.
|
|
|
(m) Wang, T.; Han, X.; Zhong, F.; Yao, W.; Lu, Y. Acc. Chem. Res. 2016, 49, 1369.
doi: 10.1021/acs.accounts.6b00163 |
|
|
(n) Li, W.; Zhang, J. Chem. Soc. Rev. 2016, 45, 1657.
doi: 10.1039/C5CS00469A |
|
|
(o) Ni, H.; Chan, W.-L.; Lu, Y. Chem. Rev. 2018, 118, 9344.
doi: 10.1021/acs.chemrev.8b00261 |
|
|
(p) Guo, H.; Fan, Y. C.; Sun, Z.; Wu, Y.; Kwon, O. Chem. Rev. 2018, 118, 10049.
doi: 10.1021/acs.chemrev.8b00081 |
|
| [34] |
(a) White, D. A.; Baizer, M. M. Tetrahedron Lett. 1973, 14, 3597.
doi: 10.1016/S0040-4039(01)86980-X pmid: 23339132 |
|
(b) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 10819.
doi: 10.1021/ja00102a071 pmid: 23339132 |
|
|
(c) Trost, B. M.; Li, C.-J. J. Am. Chem. Soc. 1994, 116, 3167.
doi: 10.1021/ja00086a074 pmid: 23339132 |
|
|
(d) Trost, B. M.; Dake, G. R. J. Org. Chem. 1997, 62, 5670.
doi: 10.1021/jo970848e pmid: 23339132 |
|
|
(e) Zhang, C.; Lu, X. Synlett 1995, 1995, 645.
doi: 10.1055/s-1995-5042 pmid: 23339132 |
|
|
(f) Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2009, 48, 2225.
doi: 10.1002/anie.200805377 pmid: 23339132 |
|
|
(g) Smith, S. W.; Fu, G. C. J. Am. Chem. Soc. 2009, 131, 14231.
doi: 10.1021/ja9061823 pmid: 23339132 |
|
|
(h) Sun, J.; Fu, G. C. J. Am. Chem. Soc. 2010, 132, 4568.
doi: 10.1021/ja101251d pmid: 23339132 |
|
|
(i) Lundgren, R. J.; Wilsily, A.; Marion, N.; Ma, C.; Chung, Y. K.; Fu, G. C. Angew. Chem., Int. Ed. 2013, 52, 2525.
doi: 10.1002/anie.201208957 pmid: 23339132 |
|
|
(j) Chen, Z.; Zhu, G.; Jiang, Q.; Xiao, D.; Cao, P.; Zhang, X. J. Org. Chem. 1998, 63, 5631.
doi: 10.1021/jo9721756 pmid: 23339132 |
|
| [35] |
(a) Zhong, F.; Dou, X.; Han, X.; Yao, W.; Zhu, Q.; Meng, Y.; Lu, Y. Angew. Chem., Int. Ed. 2013, 52, 943.
doi: 10.1002/anie.201208285 |
|
(b) Wang, T.; Yao, W.; Zhong, F.; Pang, G. H.; Lu, Y. Angew. Chem., Int. Ed. 2014, 126, 3008.
doi: 10.1002/ange.201307757 |
|
|
(c) Wang, T.; Yu, Z.; Hoon, D. L.; Huang, K.-W.; Lan, Y.; Lu, Y. Chem. Sci. 2015, 6, 4912.
doi: 10.1039/C5SC01614B |
|
|
(d) Wang, T.; Hoon, D. L.; Lu, Y. Chem. Commun. 2015, 51, 10186.
doi: 10.1039/C5CC03289J |
|
|
(e) Huang, G.; Ren, X.; Jiang, C.; Wu, J.-H.; Gao, G.; Wang, T. Org. Chem. Front. 2019, 6, 2872.
doi: 10.1039/C9QO00478E |
|
| [36] |
Wang, X.; Fang, F.; Zhao, C.; Tian, S.-K. Tetrahedron Lett. 2008, 49, 6442.
doi: 10.1016/j.tetlet.2008.08.092 |
| [37] |
(a) Wang, H.-Y.; Zhang, K.; Zheng, C.-W.; Chai, Z.; Cao, D.-D.; Zhang, J.-X.; Zhao, G. Angew. Chem., Int. Ed. 2015, 54, 1775.
doi: 10.1002/anie.201409342 |
|
(b) Lou, Y.-P.; Zheng, C.-W.; Pan, R.-M.; Jin, Q.-W.; Zhao, G.; Li, Z. Org. Lett. 2015, 17, 688.
doi: 10.1021/ol503712m |
|
|
(c) Xu, L.; Wang, H.; Zheng, C.; Zhao, G. Adv. Synth. Catal. 2017, 359, 2942.
doi: 10.1002/adsc.201700321 |
|
|
(d) Pan, R.; Zhang, J.; Zheng, C.; Wang, H.; Cao, D.; Cao, W.; Zhao, G. Tetrahedron 2017, 73, 2349.
doi: 10.1016/j.tet.2017.03.005 |
|
|
(e) Ji, X.; Cao, W.-G.; Zhao, G. Tetrahedron 2017, 73, 5983.
doi: 10.1016/j.tet.2017.08.031 |
|
|
(f) Jin, Q.; Zheng, C.; Zhao, G.; Zou, G. Tetrahedron 2018, 74, 4134.
doi: 10.1016/j.tet.2018.06.030 |
|
|
(g) Wang, H.-Y.; Zheng, C.-W.; Chai, Z.; Zhang, J.-X.; Zhao, G. Nat. Commun. 2016, 7, 12720.
doi: 10.1038/ncomms12720 |
|
| [38] |
Zhang, H.; Jiang, C.; Tan, J.-P.; Hu, H.-L.; Chen, Y.; Ren, X.; Zhang, H.-S.; Wang, T. ACS Catal. 2020, 10, 5698.
doi: 10.1021/acscatal.0c01079 |
| [39] |
(a) Fleming, F. F. Nat. Prod. Rep. 1999, 16, 597.
doi: 10.1039/a804370a |
|
(b) Miller, J. S.; Manson, J. L. Acc. Chem. Res. 2001, 34, 563.
doi: 10.1021/ar0000354 |
|
|
(c) Lindquist, B. A.; Furse, K. E.; Corcelli, S. A. Phys. Chem. 2009, 11, 8119.
|
|
| [40] |
(a) Laine, D.; Palovich, M.; McCleland, B.; Petitjean, E.; Delhom, I.; Xie, H.; Deng, J.; Lin, G.; Davis, R.; Jolit, A.; Nevins, N.; Zhao, B.; Villa, J.; Schneck, J.; McDevitt, P.; Midgett, R.; Kmett, C.; Umbrecht, S.; Peck, B.; Davis, A.-B.; Bettoun, D. ACS Med. Chem. Lett. 2011, 2, 142.
doi: 10.1021/ml100212k pmid: 30423248 |
|
(b) Raja, V.-P.-A.; Perumal, S.; Yogeeswari, P.; Sriram, D. Bioorg. Med. Chem. Lett. 2011, 21, 3881.
doi: 10.1016/j.bmcl.2011.05.032 pmid: 30423248 |
|
|
(c) Casimiro-Garcia, A.; Trujillo, J.-I.; Vajdos, F.; Juba, B.; Banker, M.-E.; Aulabaugh, A.; Balbo, P.; Bauman, J.; Chrencik, J.; Coe, J.-W.; Czerwinski, R.; Dowty, M.; Knafels, J.-D.; Kwon, S.; Leung, L.; Liang, S.; Robinson, R.-P.; Telliez, J.-B.; Unwalla, R.; Yang, X.; Thorarensen, A. J. Med. Chem. 2018, 61, 10665.
doi: 10.1021/acs.jmedchem.8b01308 pmid: 30423248 |
|
| [41] |
Oyamada, Y.; Inaba, K.; Sasamori, T.; Nakamura, S. Chem. Commun. 2022, 58, 2172.
doi: 10.1039/D1CC07191B |
| [42] |
Lubkoll, J.; Wennemers, H. Angew. Chem., Int. Ed. 2007, 46, 6841.
doi: 10.1002/anie.200702187 |
| [43] |
Bae, H. Y.; Some, S.; Lee, J. H.; Kim, J.-Y.; Song, M. J.; Lee, S.; Zhang, Y. J.; Song, C. E. Adv. Synth. Catal. 2011, 353, 3196.
doi: 10.1002/adsc.201100458 |
| [44] |
(a) Chiu, P.; Leung, L. T.; Ko, B. C. B. Nat. Prod. Rep. 2010, 27, 1066.
doi: 10.1039/b906520m pmid: 18389141 |
|
(b) Florence, G. J.; Gardnerb, N. M.; Paterson, I. Nat. Prod. Rep. 2008, 25, 342.
doi: 10.1039/b705661n pmid: 18389141 |
|
|
(c) Boucard, V.; Broustal, G.; Campagne, J. M. Eur. J. Org. Chem. 2007, 225.
pmid: 18389141 |
|
|
(d) Mondon, M.; Gesson, J.-P. Curr. Org. Synth. 2006, 3, 41.
doi: 10.2174/157017906775473966 pmid: 18389141 |
|
| [45] |
Ren, Q.; Sun, S.; Huang, J.; Li, W.; Wu, M.; Guo, H.; Wang, J.: Chem. Commun. 2014, 50, 6137.
doi: 10.1039/C4CC01736F |
| [46] |
Clerici, P.; Wennemers, H. Org. Biomol. Chem. 2012, 10, 110.
doi: 10.1039/c1ob06638b pmid: 22068641 |
| [47] |
Kolarovic, A.; Käslin, A.; Wennemers, H. Org. Lett. 2014, 16, 4236.
doi: 10.1021/ol501936n pmid: 25100030 |
| [48] |
Ling, T.; Rivas, F. Tetrahedron 2016, 72, 6729.
doi: 10.1016/j.tet.2016.09.002 |
| [49] |
(a) Corey, E. J.; Guzman-Perez, A. Angew. Chem., Int. Ed. 1998, 37, 388.
doi: 10.1002/(SICI)1521-3773(19980302)37:4【-逻*辑*与-】#x00026;lt;388::AID-ANIE388【-逻*辑*与-】#x00026;gt;3.0.CO;2-V |
|
(b) Quasdorf, K. W.; Overman, L. E. Nature 2014, 516, 181.
doi: 10.1038/nature14007 |
|
|
(c) Liu, Y.; Han, S. J.; Liu, W. B.; Stoltz, B. M. Acc. Chem. Res. 2015, 48, 740.
doi: 10.1021/ar5004658 |
|
|
(d) Li, C.; Ragab, S. S.; Liu, G.; Tang, W. Nat. Prod. Rep. 2020, 37, 276.
doi: 10.1039/C9NP00039A |
|
| [50] |
(a) Arakawa, Y.; Fritz, S. P.; Wennemers, H. J. Org. Chem. 2014, 79, 3937.
doi: 10.1021/jo500403q |
|
(b) Musa, M. A.; Cooperwood, J. S.; Khan, M. O. F. Curr. Med. Chem. 2008, 15, 2664.
doi: 10.2174/092986708786242877 |
|
|
(c) Asai, F.; Iinuma, M.; Tanaka, T.; Takenaka, M.; Mizuno, M. Phytochemistry 1992, 31, 2487.
doi: 10.1016/0031-9422(92)83306-J |
|
| [51] |
Sridharan, V.; Suryavanshi, P. A.; Menéndez, J. C. Chem. Rev. 2011, 111, 7157.
doi: 10.1021/cr100307m |
| [52] |
Engl, O. D.; Fritz, S. P.; Käslin, A.; Wennemers, H. Org. Lett. 2014, 16, 5454.
doi: 10.1021/ol502697s |
| [53] |
Cosimi, E.; Saadi, J.; Wennemers, H. Org. Lett. 2016, 18, 6014.
pmid: 27934387 |
| [54] |
(a) He, M.; Uc, G. J.; Bode, J. W. J. Am. Chem. Soc. 2006, 128, 15088.
doi: 10.1021/ja066380r pmid: 22468232 |
|
(b) Kaeobamrung, J.; Mahatthananchai, J.; Zheng, P.; Bode, J. W. J. Am. Chem. Soc. 2010, 132, 8810.
doi: 10.1021/ja103631u pmid: 22468232 |
|
|
(c) Belmessieri, D.; Morrill, L. C.; Simal, C.; Slawin, A. M.; Smith, A. D. J. Am. Chem. Soc. 2011, 133, 2714.
doi: 10.1021/ja109975c pmid: 22468232 |
|
|
(d) Mahatthananchai, J.; Kaeobamrung, J.; Bode, J. W. ACS Catal. 2012, 2, 494.
pmid: 22468232 |
|
|
(e) Morrill, L. C.; Douglas, J.; Lebl, T.; Slawin, A. M.; Fox, D. J.; Smith, A. D. Chem. Sci. 2013, 4, 4146.
doi: 10.1039/c3sc51791h pmid: 22468232 |
|
|
(f) Yao, W.; Dou, X.; Lu, Y. J. Am. Chem. Soc. 2015, 137, 54.
doi: 10.1021/ja5109358 pmid: 22468232 |
|
|
(g) Young, C. M.; Stark, D. G.; West, T. H.; Taylor, J. E.; Smith, A. D. Angew. Chem., Int. Ed. 2016, 55, 14394.
doi: 10.1002/anie.201608046 pmid: 22468232 |
|
|
(h) Zhang, M. L.; Wu, Z. J.; Zhao, J. Q.; Luo, Y.; Xu, X. Y.; Zhang, X. M.; Yuan, W. C. Org. Lett. 2016, 18, 5110.
doi: 10.1021/acs.orglett.6b02558 pmid: 22468232 |
|
|
(i) Xu, D.; Zhang, Y.; Ma, D. Tetrahedron Lett. 2010, 51, 3827.
doi: 10.1016/j.tetlet.2010.05.077 pmid: 22468232 |
|
|
(j) Sinha, D.; Perera, S.; Zhao, J. C. G. Chem. Eur. J. 2013, 19, 6976.
doi: 10.1002/chem.201300168 pmid: 22468232 |
|
| [55] |
Jin, H.; Lee, J.; Shi, H.; Lee, J. Y.; Yoo, E. J.; Song, C. E.; Ryu, D. H. Org. Lett. 2018, 20, 1584.
doi: 10.1021/acs.orglett.8b00331 |
| [56] |
Bahlinger, A.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2014, 53, 8779.
doi: 10.1002/anie.201310532 pmid: 24644150 |
| [57] |
Cosimi, E.; Engl, O. D.; Wennemers, H. Angew. Chem., Int. Ed. 2016, 55, 13127.
doi: 10.1002/anie.201607146 pmid: 27632946 |
| [58] |
(a) Mitsunuma, H.; Shibasaki, M.; Kanai, M. Angew. Chem., Int. Ed. 2012, 51, 5217.
doi: 10.1002/anie.201201132 pmid: 20063336 |
|
(b) Shimizu, Y.; Kanai, M.; Shibasaki, M. Angew. Chem., Int. Ed. 2010, 49, 1103.
doi: 10.1002/anie.200906678 pmid: 20063336 |
|
|
(c) Overman, L. E.; Paone, D. V.; Stearns, B. A. J. Am. Chem. Soc. 1999, 121, 7702.
doi: 10.1021/ja991714g pmid: 20063336 |
|
|
(d) Lemieux, R. M.; Meyers, A. I. J. Am. Chem. Soc. 1998, 120, 5453.
doi: 10.1021/ja9804159 pmid: 20063336 |
|
| [59] |
Liu, T.; Liu, W.; Shao, Z. J. Org. Chem. 2015, 80, 4950.
doi: 10.1021/acs.joc.5b00302 |
| [60] |
Engl, D. O.; Fritz, S. P.; Wennemers, H. Angew. Chem., Int. Ed. 2015, 54, 8193.
doi: 10.1002/anie.201502976 |
| [61] |
(a) Trost, B. M.; Chung, C. K.; Pinkerton, A. B. Angew. Chem., Int. Ed. 2004, 43, 4327.
doi: 10.1002/anie.200460058 pmid: 16551097 |
|
(b) Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926.
pmid: 16551097 |
|
| [62] |
Wang, Y.; Mo, M.; Zhu, K.; Zheng, C.; Zhang, H.; Wang, W.; Shao, Z. Nat. Commun. 2015, 6, 8544.
doi: 10.1038/ncomms9544 |
| [63] |
Ye, W.; Jiang, Z.; Zhao, Y.; Goh, S. L. M.; Leow, D.; Soh, Y.-T.; Tan, C.-H. Adv. Synth. Catal. 2007, 349, 2454.
doi: 10.1002/adsc.200700326 |
| [64] |
Jiang, Z.; Yang, Y.; Pan, Y.; Zhao, Y.; Liu, H.; Tan, C.-H. Chem. Eur. J. 2009, 15, 4925.
doi: 10.1002/chem.200802601 |
| [65] |
Xu, J.; Fu, X.; Low, R.; Goh, Y.-P.; Jiang, Z.; Tan, C.-H. Chem. Commun. 2008, 5526.
|
| [66] |
(a) Okino, T.; Hoashi, Y.; Takemoto, Y. J. Am. Chem. Soc. 2003, 125, 12672.
doi: 10.1021/ja036972z |
|
(b) Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. J. Am. Chem. Soc. 2005, 127, 119.
doi: 10.1021/ja044370p |
|
| [67] |
Jin, H.; Kim, S. T.; Hwang, G.-S.; Ryu, D. H. J. Org. Chem. 2016, 81, 3263.
doi: 10.1021/acs.joc.6b00218 |
| [68] |
(a) Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2017, 56, 1835.
doi: 10.1002/anie.201611466 pmid: 34163692 |
|
(b) Klijn, J. E.; Engberts, J. B. F. N. Nature 2005, 435, 746.
doi: 10.1038/435746a pmid: 34163692 |
|
|
(c) Jung, Y.; Marcus, R. A. J. Am. Chem. Soc. 2007, 129, 5492.
doi: 10.1021/ja068120f pmid: 34163692 |
|
|
(d) Guo, W.; Liu, X.; Liu, Y.; Li, C. ACS Catal. 2018, 8, 328.
doi: 10.1021/acscatal.7b02118 pmid: 34163692 |
|
|
(e) Kitanosono, T.; Kobayashi, S. Chem. Eur. J. 2020, 26, 9408.
doi: 10.1002/chem.201905482 pmid: 34163692 |
|
|
(f) Cortes-Clerget, M.; Yu, J.; Kincaid, J. R. I.; Walde, P.; Gallou, F.; Lipshutz, B. H. Chem. Sci. 2021, 12, 4237.
doi: 10.1039/d0sc06000c pmid: 34163692 |
|
| [69] |
Sim, J. H.; Park, J. H.; Maity, P.; Song, C. E. Org. Lett. 2019, 21, 6715.
doi: 10.1021/acs.orglett.9b02320 |
| [70] |
(a) Akiyama, T.; Itoh, J.; Yokota, K.; Fuchibe, K. Angew. Chem., Int. Ed. 2004, 43, 1566.
doi: 10.1002/anie.200353240 pmid: 15113196 |
|
(b) Uraguchi, D.; Terada, M. J. Am. Chem. Soc. 2004, 126, 5356.
pmid: 15113196 |
|
|
(c) Wu, X.; Li, M.; Gong, L. Acta Chim. Sinica 2013, 71, 1091. (in Chinese)
doi: 10.6023/A13030279 pmid: 15113196 |
|
|
( 吴祥, 李明丽, 龚流柱, 化学学报 2013, 71, 1091.)
doi: 10.6023/A13030279 pmid: 15113196 |
|
| [71] |
Hatano, M.; Moriyama, K.; Maki, T.; Ishihara, K. Angew. Chem., Int. Ed. 2010, 49, 3823.
doi: 10.1002/anie.201000824 |
| [72] |
Song, J.; Shih, H.-W.; Deng, L. Org. Lett. 2007, 9, 603.
pmid: 17256945 |
| [73] |
Bae, H. Y.; Kim, M. J.; Sim, J. H.; Song, C. E. Angew. Chem., Int. Ed. 2016, 55, 10825.
doi: 10.1002/anie.201605167 |
| [74] |
Gulevich, A. V.; Zhdanko, A. G.; Orru, R. V. A.; Nenajgenko, V. G. Chem. Rev. 2010, 110, 5235.
doi: 10.1021/cr900411f pmid: 20608735 |
| [75] |
Odriozola, A.; Oiarbide, M.; Palomo, C. Chem. Eur. J. 2017, 23, 12758.
doi: 10.1002/chem.201703526 |
| [76] |
Oh, J.-S.; Lee, J.-W.; Ryu, T. H.; Lee, J. H.; Song, C. E. Org. Biomol. Chem. 2012, 10, 1052.
doi: 10.1039/C1OB06629C |
| [77] |
Yoshida, Y.; Kasuya, R.; Mino, T.; Sakamoto, M. Org. Biomol. Chem. 2021, 19, 6402.
doi: 10.1039/D1OB00829C |
| [78] |
(a) Nishimura, K.; Ono, M.; Nagaoka, Y.; Tomioka, K. J. Am. Chem. Soc. 1997, 119, 12974.
doi: 10.1021/ja9729950 pmid: 23772965 |
|
(b) Nishimura, K.; Tomioka, K. J. Org. Chem. 2002, 67, 431.
pmid: 23772965 |
|
|
(c) Dong, X.-Q.; Fang, X.; Wang, C.-J. Org. Lett. 2011, 13, 4426.
doi: 10.1021/ol201766k pmid: 23772965 |
|
|
(d) Fang, X.; Li, J.; Wang, C.-J. Org. Lett. 2013, 15, 3448.
doi: 10.1021/ol4015305 pmid: 23772965 |
|
| [79] |
Rigby, C. L.; Dixon, D. J. Chem. Commun. 2008, 39, 3798.
|
| [80] |
Destro, D.; Bottinelli, C.; Ferrari, L.; Albanese, D. C. M.; Bencivenni, G.; Gillick-Healy, M. W.; Adamo, M. F. A. J. Org. Chem. 2020, 85, 5183.
doi: 10.1021/acs.joc.9b03216 pmid: 32053380 |
| [81] |
Maddocks, C. J.; Ermanis, K.; Clarke, P. A. Org. Lett. 2020, 22, 8116.
doi: 10.1021/acs.orglett.0c03090 |
| [82] |
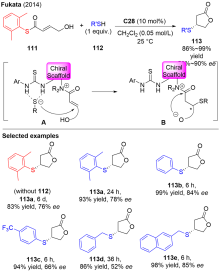
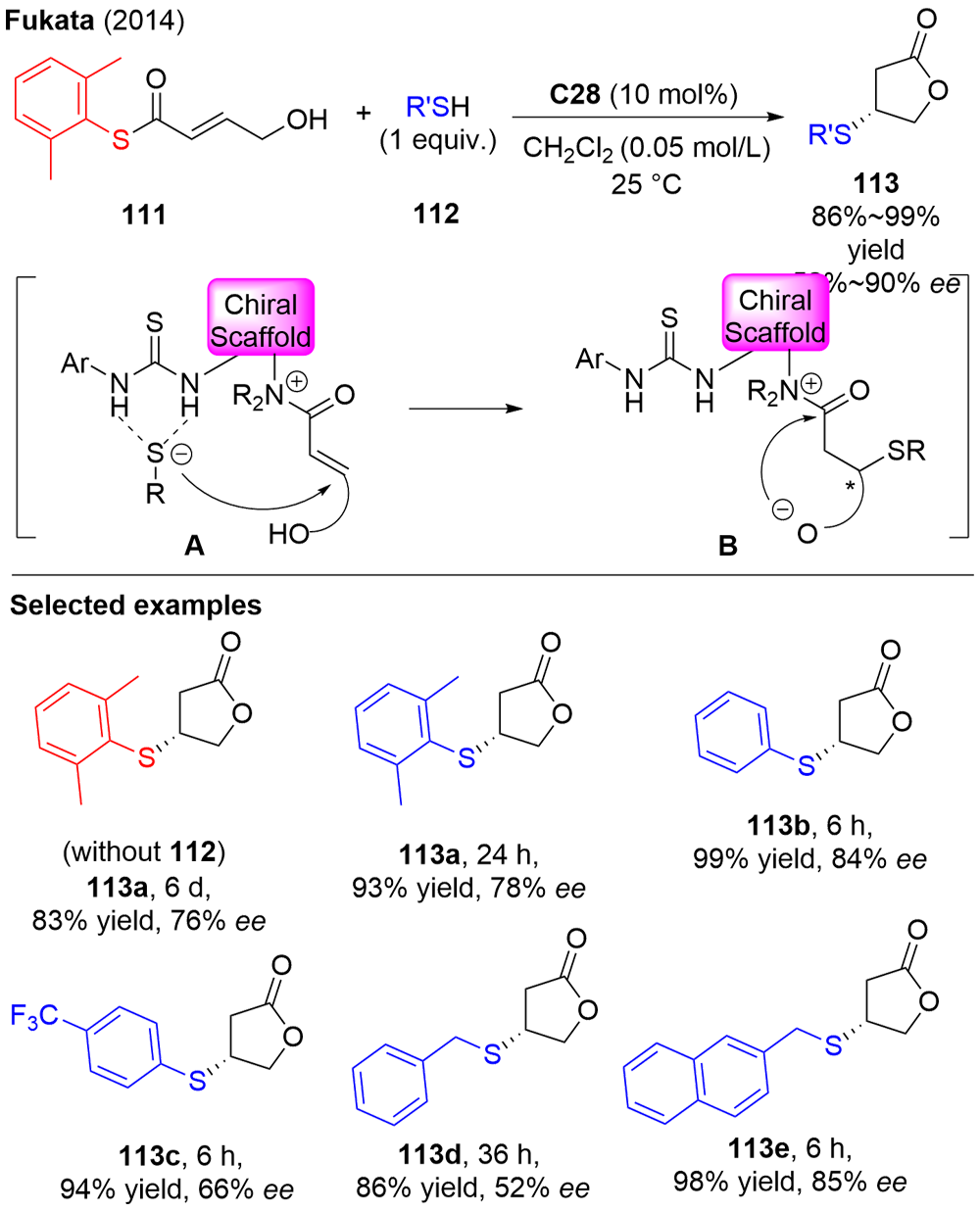
Fukata, Y.; Okamura, T.; Asano, K.; Matsubara, S. Org. Lett. 2014, 16, 2184.
doi: 10.1021/ol500637x |
| [83] |
Ahlemeyer, N. A.; Birman, V. B. Org. Lett. 2016, 18, 3454.
doi: 10.1021/acs.orglett.6b01639 pmid: 27384085 |
| [84] |
Ahlemeyer, N. A.; Streff, E. V.; Muthupandi, P.; Birman, V. B. Org. Lett. 2017, 19, 6486.
doi: 10.1021/acs.orglett.7b03044 pmid: 29200308 |
| [85] |
Lu, H.; Zhang, J.-L.; Liu, J.-Y.; Li, H.-Y.; Xu, P.-F. ACS Catal. 2017, 7, 7797.
doi: 10.1021/acscatal.7b02651 |
| [86] |
(a) Das, J. P.; Marek, I. Chem. Commun. 2011, 47, 4593.
doi: 10.1039/c0cc05222a |
|
(b) Minko, Y.; Pasco, M.; Lercher, L.; Botoshansky, M.; Marek, I. Nature 2012, 490, 522.
doi: 10.1038/nature11569 |
|
| [87] |
Giacalone, F.; Gruttaduria, M.; Agrigento, P.; Noto, R. Chem. Soc. Rev. 2012, 41, 2406.
doi: 10.1039/C1CS15206H |
| [1] | 韩明亮, 徐丽华. 过渡金属催化的硫酯的交叉偶联反应研究进展[J]. 化学学报, 2023, 81(4): 381-392. |
| [2] | 陈友根, 丁远生. 有机催化基团转移聚合的研究进展[J]. 化学学报, 2020, 78(8): 733-745. |
| [3] | 蔡倩, 马浩文. 手性高价碘试剂的发展及展望[J]. 化学学报, 2019, 77(3): 213-230. |
| [4] | 张毛毛, 骆元元, 陆良秋, 肖文精. 过渡金属与有机小分子协同催化的不对称烯丙基取代反应研究进展[J]. 化学学报, 2018, 76(11): 838-849. |
| [5] | 石明林, 詹固, 杜玮, 陈应春. 靛红衍生的硝酮与酮亚胺的不对称直接氮杂插烯Mannich反应[J]. 化学学报, 2017, 75(10): 998-1002. |
| [6] | 赵文静, 乔增莹, 段中余, 王浩. pH和氧化双响应性聚β硫酯化合物的制备及其聚集性质的研究[J]. 化学学报, 2016, 74(3): 234-240. |
| [7] | 张启超, 吕健, 罗三中. 碳正离子Lewis酸催化氧化还原中性胺基α-C(sp3)H芳基化反应[J]. 化学学报, 2016, 74(1): 61-66. |
| [8] | 王雨卉, 曹中艳, 牛艳霏, 赵小莉, 周剑. 高对映选择性的有机催化的硝基烷烃对N-Boc靛红亚胺的不对称aza-Henry反应[J]. 化学学报, 2014, 72(7): 867-872. |
| [9] | 马世雄, 钟源, 王守磊, 许兆青, 常民, 王锐. 有机催化吡唑酮与MBH碳酸酯的不对称烯丙基烷基化反应[J]. 化学学报, 2014, 72(7): 825-829. |
| [10] | 于天洋, 王瑶, 许鹏飞. 超分子亚胺离子催化的新途径[J]. 化学学报, 2014, 72(7): 845-848. |
| [11] | 孙峋皓, 彭景, 张叔阳, 周清清, 董琳, 陈应春. 靛红Morita-Baylis-Hillman碳酸酯与环状N-磺酰亚胺的不对称烯丙基烷基化反应研究[J]. 化学学报, 2012, 70(16): 1682-1685. |
| [12] | 刘运林, 周剑. 高对映选择性的有机催化的二氟烯醇硅醚与β,γ-不饱和-α-酮酸酯的不对称Mukaiyama-aldol反应[J]. 化学学报, 2012, 70(13): 1451-1456. |
| [13] | 代先东, 范崇旭, 曹瑛, 刘尚义, 蒋辉, 陈冀胜. 半胱氨酸肽片段连接法合成ω-芋螺毒素MVIIA[J]. 化学学报, 2010, 68(07): 667-671. |
| [14] | 郑吉富, 李耀先, 张锁秦, 王小明, 杨松涛, 白杰, 王芳, 张广良, 刘福安. 新颖有机催化剂的合成及催化不对称直接Aldol反应研究[J]. 化学学报, 2007, 65(6): 553-556. |
| [15] | 刘强, 张秉坚 . 石质文物表面生物矿化保护材料的仿生制备[J]. 化学学报, 2006, 64(15): 1601-1605. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||