化学学报 ›› 2023, Vol. 81 ›› Issue (1): 42-63.DOI: 10.6023/A22100419 上一篇 下一篇
综述
投稿日期:2022-10-10
发布日期:2022-11-21
基金资助:
Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou( ), Qian Cai(
), Qian Cai( )
)
Received:2022-10-10
Published:2022-11-21
Contact:
*E-mail: Supported by:文章分享
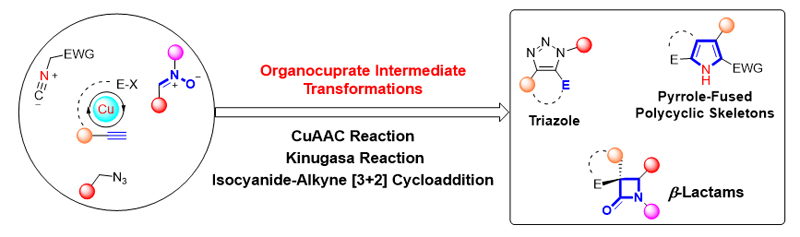
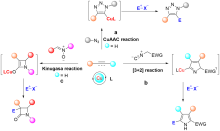
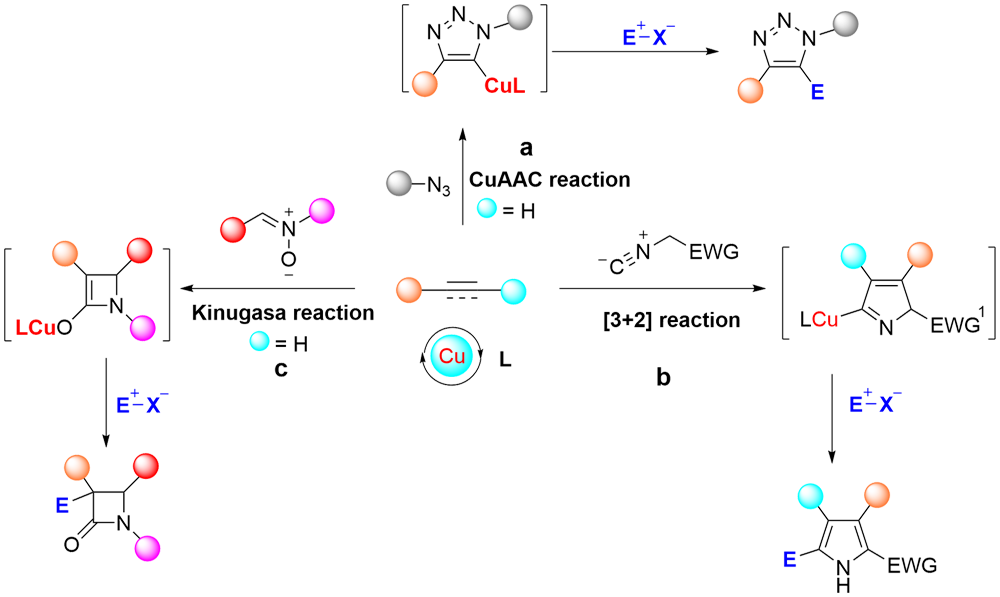
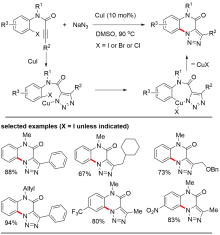
铜(I)盐催化的环加成反应, 如叠氮-炔[3+2]环加成(CuAAC)、不饱和化合物与异氰基化合物的[3+2]环加成、硝酮-炔的环加成(Kinugasa反应)是构建多类氮杂环的高效合成方法, 被广泛应用于有机合成的各个领域. 近年来, 针对几类环加成反应中产生的有机亚铜中间体的多样性转化吸引了国内外很多课题组的注意, 基于对这些环加成反应中有机亚铜中间体的捕捉, 多类串联及多组分反应得以发展, 从而成功实现了一系列多取代杂环或稠环结构的高效构建. 本综述总结了这一领域的研究进展, 按照所经历的有机亚铜中间体的类型进行分类, 包括: (1) CuAAC反应中产生的三氮唑亚铜中间体; (2) 炔烃与异氰化合物[3+2]环加成反应中产生的2H-吡咯基亚铜中间体; (3) Kinugasa反应中产生的烯醇亚铜中间体. 期望此综述能够有助于研究者了解有机亚铜中间体捕捉策略的发展、应用现状及不足之处, 进一步推动铜催化转化的发展.
邱孔茜, 李杰, 马浩文, 周伟, 蔡倩. 捕捉环加成反应中的有机亚铜中间体构筑氮杂环化合物研究进展[J]. 化学学报, 2023, 81(1): 42-63.
Kongxi Qiu, Jie Li, Haowen Ma, Wei Zhou, Qian Cai. Recent Advances in the Construction of Nitrogen-Containing Heterocycles via Trapping Organocopper(I) Intermediates[J]. Acta Chimica Sinica, 2023, 81(1): 42-63.
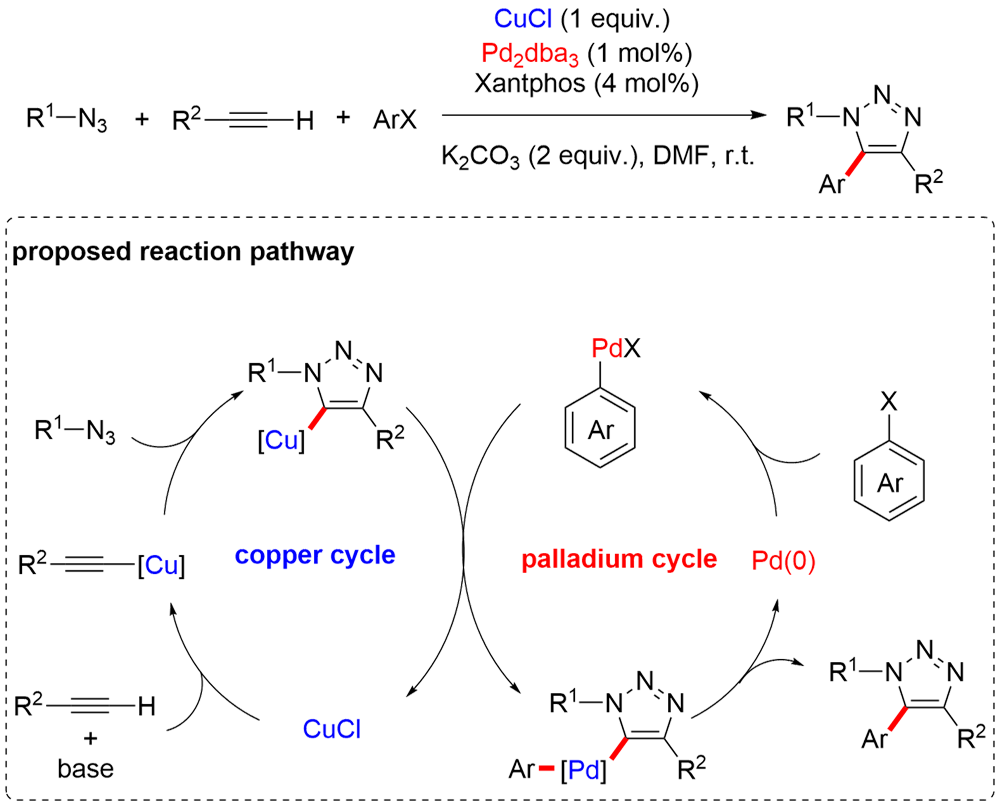

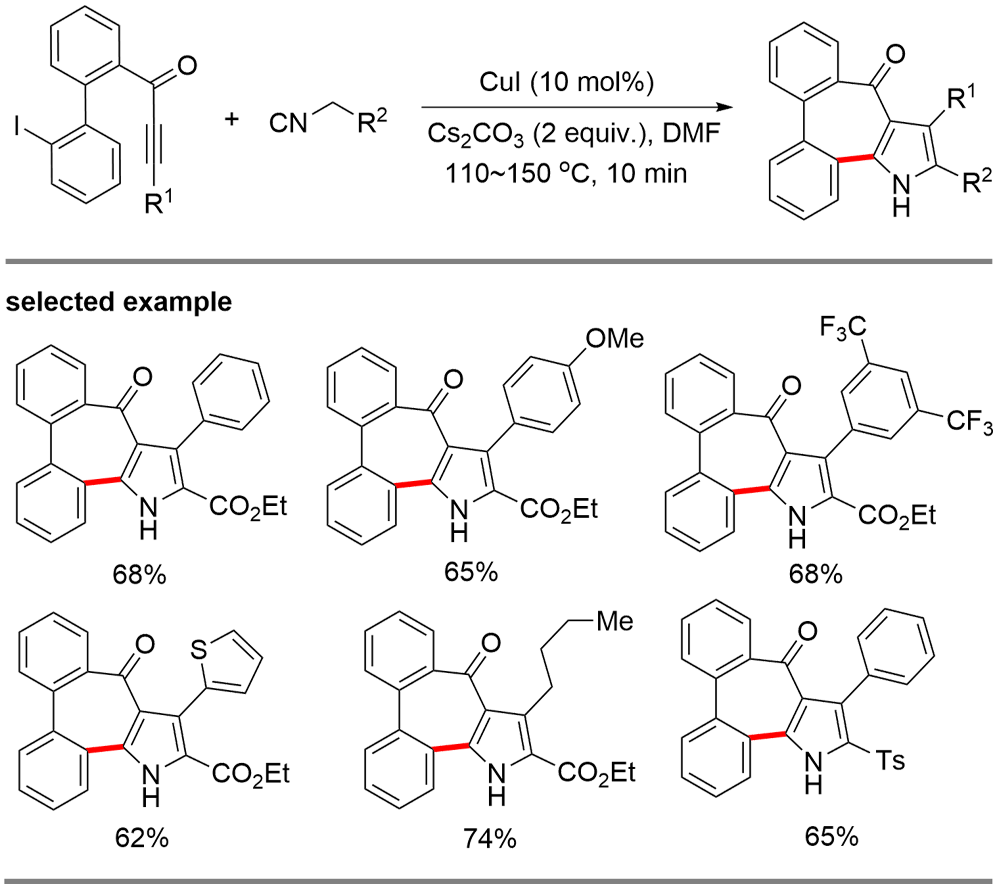
| [1] |
Gilman, H.; Jones, R. G.; Woods, L. A. J. Org. Chem. 1952, 17, 1630.
doi: 10.1021/jo50012a009 |
| [2] |
(a) Krause, N. Modern Organocopper Chemistry, Wiley-VCH, Weinheim, Germany, 2002.
pmid: 22111574 |
|
(b) Woodward, S. Chem. Soc. Rev. 2000, 29, 393.
doi: 10.1039/b002690p pmid: 22111574 |
|
|
(c) Yoshikai, N.; Nakamura, E. Chem. Rev. 2012, 112, 2339.
doi: 10.1021/cr200241f pmid: 22111574 |
|
|
(d) Tang, H.; Huang, D.; Li, Y.; Du, X.; Lian, H.; Chen, J. Acta Chim. Sinica 1994, 52, 306. (in Chinese)
pmid: 22111574 |
|
|
( 唐洪春, 黄道孝, 李玉林, 杜秀宝, 连洪寿, 陈冀胜, 化学学报 1994, 52, 306.)
pmid: 22111574 |
|
| [3] |
Kharasch, M. S.; Tawney, P. O. J. Am. Chem. Soc. 1941, 63, 2308.
doi: 10.1021/ja01854a005 |
| [4] |
For selected reviews, see: (a) Reymond, S.; Cossy, J. Chem. Rev. 2008, 108, 5359.
doi: 10.1021/cr078346g pmid: 25961125 |
|
(b) Stanley, L. M.; Sibi, M. P. Chem. Rev. 2008, 108, 2887.
doi: 10.1021/cr078371m pmid: 25961125 |
|
|
(c) Hein, J. E.; Fokin, V. V. Chem. Soc. Rev. 2010, 39, 1302.
doi: 10.1039/b904091a pmid: 25961125 |
|
|
(d) Khangaro, R. K.; Kaliappan, K. P. Eur. J. Org. Chem. 2013, 7664.
pmid: 25961125 |
|
|
(e) Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366.
doi: 10.1021/cr5007182 pmid: 25961125 |
|
|
(f) Haldón, E.; Nicasio, M. C.; Pérez, P. J. Org. Biomol. Chem. 2015, 13, 9528.
doi: 10.1039/C5OB01457C pmid: 25961125 |
|
| [5] |
(a) Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057.
doi: 10.1021/jo011148j |
|
(b) Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596.
doi: 10.1002/1521-3773(20020715)41:14【-逻*辑*与-】#x00026;lt;2596::AID-ANIE2596【-逻*辑*与-】#x00026;gt;3.0.CO;2-4 |
|
|
(c) Dong, J.; Xu, L. Chin. J. Chem. 2020, 38, 414.
doi: 10.1002/cjoc.201900421 |
|
| [6] |
(a) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 565.
|
|
(b) Huisgen, R. Angew. Chem., Int. Ed. 1963, 2, 633.
|
|
|
(c) Huisgen, R. Pure Appl. Chem. 1989, 61, 613.
doi: 10.1351/pac198961040613 |
|
| [7] |
For selected reviews, see: (a) Meldal, M.; Tornøe, C. W. Chem. Rev. 2008, 108, 2952.
doi: 10.1021/cr0783479 pmid: 26796328 |
|
(b) Tiwari, V. K.; Mishra, B. B.; Mishra, K. B.; Mishra, N.; Singh, A. S.; Chen, X. Chem. Rev. 2016, 116, 3086.
doi: 10.1021/acs.chemrev.5b00408 pmid: 26796328 |
|
|
(c) Wang, X.; Huang, B.; Liu, X.; Zhan, P. Drug Discov. Today 2016, 21, 118.
doi: 10.1016/j.drudis.2015.08.004 pmid: 26796328 |
|
|
(d) Döhler, D.; Michael, P.; Binder, W. H. Acc. Chem. Res. 2017, 50, 2610.
doi: 10.1021/acs.accounts.7b00371 pmid: 26796328 |
|
|
(e) Meldal, M.; Diness, F. Trends Chem. 2020, 2, 569.
doi: 10.1016/j.trechm.2020.03.007 pmid: 26796328 |
|
| [8] |
Nolte, C.; Mayer, P.; Straub, B. F. Angew. Chem., Int. Ed. 2007, 46, 2101.
doi: 10.1002/anie.200604444 |
| [9] |
Wu, Y.-M.; Deng, J.; Li, Y.; Chen, Q.-Y. Synthesis 2005, 8, 1314.
|
| [10] |
Cassidy, M. P.; Raushel, J.; Fokin, V. V. Angew. Chem., Int. Ed. 2006, 45, 3154.
doi: 10.1002/anie.200503805 |
| [11] |
Zhang, X.; Hsung, R. P.; Li, H. Chem. Commun. 2007, 2420.
|
| [12] |
Li, L.; Zhang, G.; Zhu, A.; Zhang, L. J. Org. Chem. 2008, 73, 3630.
doi: 10.1021/jo800035v |
| [13] |
Li, L.; Li, R.; Zhu, A.; Zhang, G.; Zhang, L. Synlett 2011, 874.
|
| [14] |
Malnuit, V.; Duca, M.; Manout, A.; Bougrin, K.; Benhida, R. Synlett 2009, 2123.
|
| [15] |
(a) Wang, B.; Ahmed, M. N.; Zhang, J.; Chen, W.; Wang, X.; Hu, Y. Tetrahedron Lett. 2013, 54, 6097.
doi: 10.1016/j.tetlet.2013.08.121 pmid: 24032422 |
|
(b) Wang, B.; Zhang, J.; Wang, X.; Liu, N.; Chen, W.; Hu, Y. J. Org. Chem. 2013, 78, 10519.
doi: 10.1021/jo401629x pmid: 24032422 |
|
|
(c) Wang, B.; Liu, N.; Shao, C.; Zhang, Q.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2013, 355, 2564.
doi: 10.1002/adsc.201300307 pmid: 24032422 |
|
|
(d) Wang, B.; Liu, N.; Chen, W.; Huang, D.; Wang, X.; Hu, Y. Adv. Synth. Catal. 2015, 357, 401.
doi: 10.1002/adsc.201400471 pmid: 24032422 |
|
| [16] |
Yan, R.; Sander, K.; Galante, E.; Rajkumar, V.; Badar, A.; Robson, M.; El-Emir, E.; Lythgoe, M. F.; Pedley, R. B.; Årstad, E. J. Am. Chem. Soc. 2013, 135, 703.
doi: 10.1021/ja307926g |
| [17] |
Chen, Z.; Zhu, J.; Xie, H.; Li, S.; Wu, Y.; Gong, Y. Adv. Synth. Catal. 2010, 352, 1296.
doi: 10.1002/adsc.200900875 |
| [18] |
Cai, Q.; Zhou, W. Chin. J. Chem. 2020, 38, 879.
doi: 10.1002/cjoc.202000075 |
| [19] |
Yan, J.; Zhou, F.; Qin, D.; Cai, T.; Ding, K.; Cai, Q. Org. Lett. 2012, 14, 1262.
doi: 10.1021/ol300114w |
| [20] |
Hooyberghs, G.; De Coster, H.; Vachhani, D. D.; Ermolat’ev, D. S.; Van der Eycken, E. V. Tetrahedron 2013, 69, 4331.
doi: 10.1016/j.tet.2013.03.031 |
| [21] |
Vachhani, D. D.; Kumar, A.; Modha, S. G.; Sharma, S. K.; Parmar, V. S.; Van der Eycken, E. V. Eur. J. Org. Chem. 2013, 1223.
|
| [22] |
Reddy, A. S.; Reddy, M. N.; Swamy, K. C. K. RSC Adv. 2014, 4, 28359.
doi: 10.1039/C4RA03503H |
| [23] |
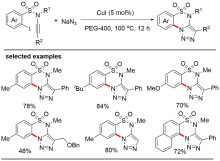
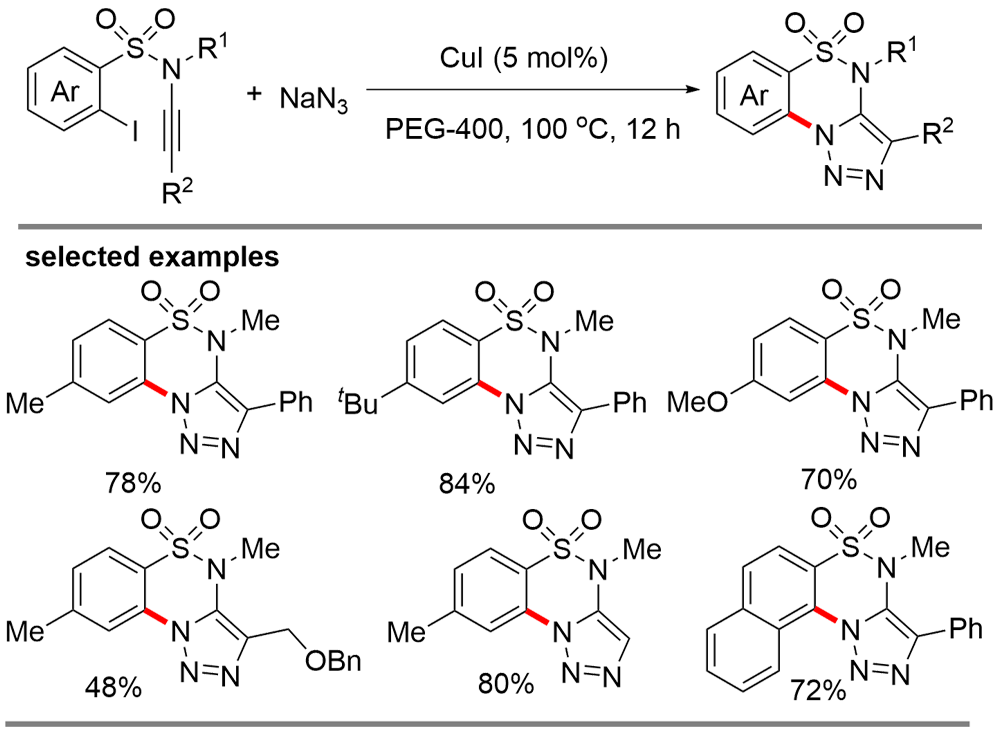
An, Y.; He, H.; Liu, T.; Zhang, Y.; Lu, X.; Cai, Q. Synthesis 2017, 49, 3863.
doi: 10.1055/s-0036-1590791 |
| [24] |
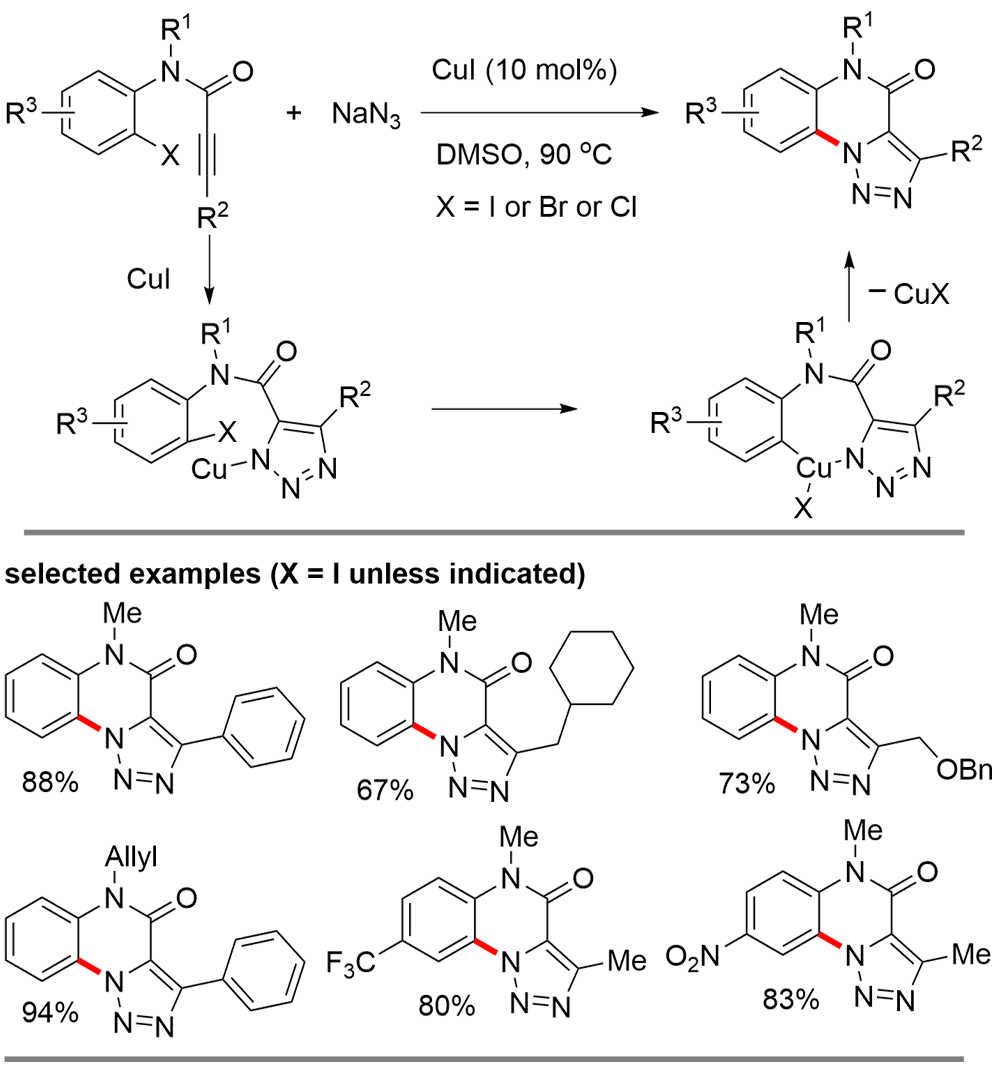
Xiao, G.; Wu, K.; Zhou, W.; Cai, Q. Adv. Synth. Catal. 2021, 363, 4988.
doi: 10.1002/adsc.202100955 |
| [25] |
Cai, Q.; Yan, J.; Ding, K. Org. Lett. 2012, 14, 3332.
doi: 10.1021/ol301307x |
| [26] |
Ouyang, Y.; Si, H.; Zhu, C.; Zhong, L.; Ma, H.; Li, Z.; Xiong, H.; Liu, T.; Liu, Z.; Zhang, Z.; Zhang, Z.; Cai, Q. J. Med. Chem. 2022, 65, 7833.
doi: 10.1021/acs.jmedchem.2c00271 |
| [27] |
Bag, S. S.; Das, S. K.; Gogoi, H. Tetrahedron, 2018, 74, 2218.
doi: 10.1016/j.tet.2018.03.037 |
| [28] |
Reddy, M. N.; Swamy, K. C. K. Eur. J. Org. Chem. 2012, 2013.
|
| [29] |
Wei, F.; Wang, W.; Ma, Y.; Tung, C.-H.; Xu, Z. Chem. Commun. 2016, 52, 14188.
doi: 10.1039/C6CC06194J |
| [30] |
Ackermann, L.; Potukuchi, H. K.; Landsberg, D.; Vicente, R. Org. Lett. 2008, 10, 3081.
doi: 10.1021/ol801078r pmid: 18549230 |
| [31] |
Qian, W.; Wang, H.; Allen, J. Angew. Chem., Int. Ed. 2013, 52, 10992.
doi: 10.1002/anie.201305970 |
| [32] |
Pericherla, K.; Jha, A.; Khungar, B.; Kumar, A. Org. Lett. 2013, 15, 4304.
doi: 10.1021/ol401655r |
| [33] |
Liu, Z.; Zhu, D.; Luo, B.; Zhang, N.; Liu, Q.; Hu, Y.; Pi, R.; Huang, P.; Wen, S. Org. Lett. 2014, 16, 5600.
doi: 10.1021/ol502654a |
| [34] |
Lautens, M.; Larin, E. M. Angew. Chem., Int. Ed. 2019, 58, 13438.
doi: 10.1002/anie.201907448 |
| [35] |
Wei, F.; Li, H.; Song, C.; Ma, Y.; Zhou, L.; Tung, C.-H.; Xu, Z. Org. Lett. 2015, 17, 2860.
doi: 10.1021/acs.orglett.5b01342 |
| [36] |
(a) Zhang, Z.; Zhou, Q.; Ye, F.; Xia, Y.; Wu, G.; Hossain, M. L.; Zhang, Y.; Wang, J. Adv. Synth. Catal. 2015, 357, 2277.
doi: 10.1002/adsc.201500377 |
|
(b) Zhang, Z.; Zhou, Q.; Yu, W.; Li, T.; Zhang, Y.; Wang, J. Chin. J. Chem. 2017, 35, 387.
doi: 10.1002/cjoc.201600888 |
|
| [37] |
Chen, F.-J.; Mamidipalli, P.; Sabbasani, V. R.; Liu, H.; Xia, Y.; Lee, D. Org. Chem. Front. 2021, 8, 6095.
doi: 10.1039/D1QO01112J |
| [38] |
Wang, W.; Wei, F.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2016, 18, 4158.
doi: 10.1021/acs.orglett.6b02199 |
| [39] |
(a) Zhou, W.; Zhang, M.; Li, H.; Chen, W. Org. Lett. 2017, 19, 10.
doi: 10.1021/acs.orglett.6b02850 pmid: 27966996 |
|
(b) Wu, F.; Zhou, W.; Chen, K.; Chen, W.; Liu, M.; Wu, H. Adv. Synth. Catal. 2018, 360, 2435.
doi: 10.1002/adsc.201800394 pmid: 27966996 |
|
| [40] |
Cheung, K. P. S.; Tsui, G. C. Org. Lett. 2017, 19, 2881.
doi: 10.1021/acs.orglett.7b01116 |
| [41] |
Wu, W.; Wang, J.; Wang, Y.; Huang, Y.; Tan, Y.; Weng, Z. Angew. Chem., Int. Ed. 2017, 56, 10476.
doi: 10.1002/anie.201705620 |
| [42] |
Wang, W.; Peng, X.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2016, 55, 649.
doi: 10.1002/anie.201509124 |
| [43] |
Wang, W.; Huang, S.; Yan, S.; Sun, X.; Tung, C.-H.; Xu, Z. Chin. J. Chem. 2020, 38, 445.
doi: 10.1002/cjoc.201900556 |
| [44] |
Reddy, R. J.; Waheed, M.; Kumari, A. H.; Krishna, G. R. Adv. Synth. Catal. 2022, 364, 319.
doi: 10.1002/adsc.202101256 |
| [45] |
Wei, F.; Zhou, T.; Ma, Y.; Tung, C.-H.; Xu, Z. Org. Lett. 2017, 19, 2098.
doi: 10.1021/acs.orglett.7b00701 |
| [46] |
Yang, D.; Fu, N.; Liu, Z.; Li, Y.; Chen, B. Synlett 2007, 278.
|
| [47] |
Alonso, F.; Moglie, Y.; Radivoy, G.; Yus, M. Synlett 2012, 23, 2179.
doi: 10.1055/s-0031-1290445 |
| [48] |
Li, L.; Hao, G.; Zhu, A.; Fan, X.; Zhang, G.; Zhang, L. Chem. Eur. J. 2013, 19, 14403.
doi: 10.1002/chem.201303324 |
| [49] |
(a) Li, L.; Fan, X.; Zhang, Y.; Zhu, A.; Zhang, G. Tetrahedron 2013, 69, 9939.
doi: 10.1016/j.tet.2013.09.093 |
|
(b) Li, L.-J.; Zhang, Y.-Q.; Zhang, Y.; Zhu, A.-L.; Zhang, G.-S. Chin. Chem. Lett. 2014, 25, 1161.
doi: 10.1016/j.cclet.2014.03.004 |
|
| [50] |
Selvaraju, M.; Sun, C.-M. Adv. Synth. Catal. 2014, 356, 1329.
doi: 10.1002/adsc.201301013 |
| [51] |
(a) Peringer, F.; do Nascimento, J. E. R.; Abib, P. B.; Barcellos, T.; Van der Eycken, E. V.; Perin, G.; Jacob, R. G. Alves, D. Eur. J. Org. Chem. 2017, 2579.
|
|
(b) Aquino, T. B.; do Nascimento, J. E. R.; Dias, Í. F. C.; de Oliveira, D. H.; Barcellos, T.; Lenardão, E. J.; Perin, G.; Alves, D.; Jacob, R. J. Tetrahedron Lett. 2018, 59, 1080.
doi: 10.1016/j.tetlet.2018.01.072 |
|
| [52] |
Xu, J.; Song, Q. Org. Chem. Front. 2017, 4, 938.
doi: 10.1039/C6QO00725B |
| [53] |
Yu, X.; Xu, J.; Zhou, Y.; Song, Q. Org. Chem. Front. 2018, 5, 2463.
doi: 10.1039/C8QO00590G |
| [54] |
Wang, X.-X.; Xin, Y.; Li, Y.; Xia, W.-J.; Zhou, B.; Ye, R.-R.; Li, Y.-M. J. Org. Chem. 2020, 85, 3576
doi: 10.1021/acs.joc.9b03285 |
| [55] |
(a) Navarro, Y.; López, J. G.; Iglesias, M. J.; Ortiz, F. L. Org. Lett. 2021, 23, 334.
doi: 10.1021/acs.orglett.0c03838 |
|
(b) Navarro, Y.; Jiménez, I. H.; Iglesias, M. J.; Ortiz, F. L. Synthesis 2022, 54, 199.
doi: 10.1055/a-1577-5999 |
|
| [56] |
(a) Kamijo, S.; Kanazawa, C.; Yamamoto, Y. J. Am. Chem. Soc. 2005, 127, 9260.
pmid: 15969607 |
|
(b) Larionov, O. V.; de Meijere, A. Angew. Chem., Int. Ed. 2005, 44, 5664.
doi: 10.1002/anie.200502140 pmid: 15969607 |
|
| [57] |
Cai, Q.; Zhou, F.; Xu, T.; Fu, L.; Ding, K. Org. Lett. 2011, 13, 340.
doi: 10.1021/ol102826f |
| [58] |
Zhou, F.; Fu, L.; Wei, J.; Ding, K.; Cai, Q. Synthesis 2011, 3037.
|
| [59] |
Zhou, F.; Liu, J. Ding, K.; Liu, J.; Cai, Q. J. Org. Chem. 2011, 76, 5346.
doi: 10.1021/jo2006939 |
| [60] |
Zheng, D.; Liu, T.; Liu, X.; Fan, X.; Wu, J. J. Org. Chem. 2016, 81, 9428.
doi: 10.1021/acs.joc.6b01669 |
| [61] |
Ouyang, Y.; Wu, K.; Zhou, W.; Cai, Q. Org. Chem. Front. 2021, 8, 2456.
doi: 10.1039/D0QO01657H |
| [62] |
Kinugasa, M.; Hashimoto, S. J. Chem. Soc. Chem. Commun. 1972, 466.
|
| [63] |
(a) Santoro, S.; Himo, F. J. Org. Chem. 2021, 86, 10665.
doi: 10.1021/acs.joc.1c01351 |
|
(b) Malig, T. C.; Yu, D.; Hein, J. E. J. Am. Chem. Soc. 2018, 140, 9167.
doi: 10.1021/jacs.8b04635 |
|
|
(c) Santoro, S.; Liao, R.-Z.; Marcelli, T.; Hammar, P.; Himo, F. J. Org. Chem. 2015, 80, 2649.
doi: 10.1021/jo502838p |
|
| [64] |
Shintani, R.; Fu, G. C. Angew. Chem., Int. Ed. 2003, 42, 4082.
doi: 10.1002/anie.200352103 |
| [65] |
Shu, T.; Zhao, L.; Li, S.; Chen, X.-Y.; von Essen, C.; Rissanen, K.; Enders, D. Angew. Chem., Int. Ed. 2018, 57, 10985.
doi: 10.1002/anie.201806931 |
| [66] |
Qi, J.; Wei, F.; Huang, S.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 4561.
doi: 10.1002/anie.202013450 |
| [67] |
(a) Qi, J.; Wei, F.; Tung, C.-H.; Xu, Z. Angew. Chem., Int. Ed. 2021, 60, 13814.
doi: 10.1002/anie.202100601 |
|
(b) Qi, J.; Tung, C.-H.; Xu, Z. Trends Chem. 2021, 3, 984.
doi: 10.1016/j.trechm.2021.08.004 |
|
| [68] |
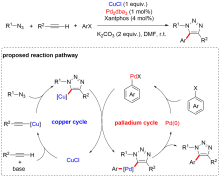
Zhong, X.; Huang, M.; Xiong, H.; Liang, Y.; Zhou, W.; Cai, Q. Angew. Chem., Int. Ed. 2022, DOI: 10.1002/anie.202208323.
doi: 10.1002/anie.202208323 |
| [1] | 罗江浩, 马浩文, 张杰豪, 周伟, 蔡倩. 串联炔-异氰[3+2]环加成/Boulton-Katritzky重排/扩环反应构建吡咯并[3,2-d]嘧啶-4-酮化合物★[J]. 化学学报, 2023, 81(8): 898-904. |
| [2] | 鱼章龙, 李忠良, 杨昌江, 顾强帅, 刘心元. 铜催化的二醇类化合物对映选择性去对称化反应研究进展★[J]. 化学学报, 2023, 81(8): 955-966. |
| [3] | 王瑞祥, 赵庆如, 顾庆, 游书力. 金/铱接力催化炔基酰胺环化/不对称烯丙基苄基化串联反应★[J]. 化学学报, 2023, 81(5): 431-434. |
| [4] | 孟庆端, 韩佳宏, 潘一骁, 郝伟, 范青华. C1-对称手性氮杂环卡宾(NHC)配体的不对称合成及其催化性能研究★[J]. 化学学报, 2023, 81(10): 1271-1279. |
| [5] | 徐清浩, 魏立谱, 张震, 肖斌. 铜促进的锗亲电试剂与烷基溴合成四烷基锗※[J]. 化学学报, 2022, 80(4): 428-431. |
| [6] | 满清敏, 付尊蕴, 刘甜甜, 郑明月, 蒋华良. Cu催化偶联反应合成烷基芳基醚的DFT机理研究[J]. 化学学报, 2021, 79(7): 948-952. |
| [7] | 邓卓基, 欧阳溢凡, 敖运林, 蔡倩. 铜催化不对称去对称化分子内烯基C—N偶联反应[J]. 化学学报, 2021, 79(5): 649-652. |
| [8] | 杨妲, 张龙力, 刘欢, 杨朝合. 双功能配体修饰的Ir催化剂在“氢甲酰化-缩醛化”串联反应中的共催化作用[J]. 化学学报, 2021, 79(5): 658-662. |
| [9] | 张荣华, 许冰, 张展鸣, 张俊良. Ming-Phos/铜催化的亚甲胺叶立德与硝基烯烃的不对称[3+2]环加成反应[J]. 化学学报, 2020, 78(3): 245-249. |
| [10] | 黄浩, 林华鑫, 王敏, 廖建. 1,2-苯基异噁唑为氮源的铜催化苯乙烯不对称硼胺化[J]. 化学学报, 2020, 78(11): 1229-1234. |
| [11] | 梁欢, 苟阿龙, 高珠鹏, 雷林生, 王博文, 余兰, 徐学涛, 王少华. 铜催化的α-氨基丙二腈的脱氰氧代反应:一种合成叔酰胺的新方法[J]. 化学学报, 2020, 78(10): 1064-1068. |
| [12] | 许健, 张世樊, 罗莹, 张荔, 张帆, 黄挺菁, 宋秋玲. 自由基促进硫甲基取代的炔酮的环化反应[J]. 化学学报, 2019, 77(9): 932-938. |
| [13] | 张衡, 牟学清, 陈弓, 何刚. 铜催化苯甲酰亚胺高烯丙酯的分子内胺化全氟烷基化反应[J]. 化学学报, 2019, 77(9): 884-888. |
| [14] | 成忠明, 陈品红, 刘国生. 光/铜共催化远程C—H键的不对称氰基化反应[J]. 化学学报, 2019, 77(9): 856-860. |
| [15] | 林凤闺蓉, 梁宇杰, 郦鑫耀, 宋颂, 焦宁. 氧气氧化铜催化的苯胺邻位叠氮化反应[J]. 化学学报, 2019, 77(9): 906-910. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||