化学学报 ›› 2024, Vol. 82 ›› Issue (2): 146-151.DOI: 10.6023/A23110498 上一篇 下一篇
研究论文
投稿日期:2023-11-14
发布日期:2024-01-17
作者简介:基金资助:
Xiaoyu Gua, Jin Lia, Qian Sunb, Chaoyang Wangb( )
)
Received:2023-11-14
Published:2024-01-17
Contact:
E-mail: About author:Supported by:文章分享
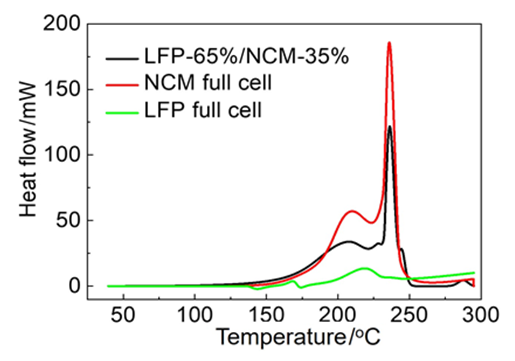
动力锂离子电池(LIB)的安全问题, 尤其是热失控这一频发的安全事件严重影响乘车人员的安全以及新能源汽车的推广. 本工作使用C80微量量热仪准确测量了商用锂离子电池的内在产热, 通过研究分析不同质量比例的负极-电解液产热及不同质量比例的正极-负极产热, 明确了LIB在热失控阶段的主要反应分为负极与电解液反应放热(130~200 ℃), 三元镍钴锰酸锂(NCM)正极释氧与负极反应放热(200~240 ℃)和磷酸铁锂(LFP)正极释氧与负极反应放热(240~300 ℃)等. 通过使用去卷积数学方法对不同质量比例的负极-电解液及不同质量比例的正极-负极产热分析研究表明, 在商用锂电池注液系数条件下, 电解液会优先与负极反应且被全部消耗, 剩余嵌锂负极会进一步与正极反应放热, 且反应热与正极材料特性密切相关. 残余正极物质虽然结构坍塌仍会释氧, 但由于缺少与之反应的负极或电解液, 热量不会再明显增加. 通过对不同荷电状态(SOC)及不同类型的锂电池主材进行产热测试, 能更好地指导电极材料的改性和电池组装的开发设计, 从而提高LIB整体热稳定性和安全性, 最终获得整包和新能源车的安全提高.
顾晓瑜, 李进, 孙千, 王朝阳. 微量量热法分析锂离子电池热失控过程[J]. 化学学报, 2024, 82(2): 146-151.
Xiaoyu Gu, Jin Li, Qian Sun, Chaoyang Wang. Microcalorimetry Analysis of Thermal Runaway Process in Lithium-ion Batteries[J]. Acta Chimica Sinica, 2024, 82(2): 146-151.


| LFP/NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 130 | 180 | 84 | 1408 |
| peak 2 | 174 | 205 | 299 | |
| peak 3 | 213 | 228 | 140 | 2293 |
| peak 4 | 231 | 236 | 267 | |
| peak 5 | 241 | 245 | 29 | |
| peak 6 | 280 | 287 | 21 | 152 |
| NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 154 | 180 | 66 | 1632 |
| peak 2 | 185 | 208 | 378 | |
| peak 3 | 211 | 224 | 294 | 1285 |
| peak 4 | 232 | 235 | 109 | |
| peak 5 | 226 | 236 | 249 | |
| peak 6 | — | — | — | |
| LFP | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 160 | 168 | 8 | 448 |
| peak 2 | 180 | 209 | 65 | |
| peak 3 | 197 | 219 | 33 | |
| peak 4 | 198 | 231 | 16 | |
| peak 5 | — | — | — | |
| peak 6 | 222 | 294 | 122 | 240 |
| LFP/NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 130 | 180 | 84 | 1408 |
| peak 2 | 174 | 205 | 299 | |
| peak 3 | 213 | 228 | 140 | 2293 |
| peak 4 | 231 | 236 | 267 | |
| peak 5 | 241 | 245 | 29 | |
| peak 6 | 280 | 287 | 21 | 152 |
| NCM | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 154 | 180 | 66 | 1632 |
| peak 2 | 185 | 208 | 378 | |
| peak 3 | 211 | 224 | 294 | 1285 |
| peak 4 | 232 | 235 | 109 | |
| peak 5 | 226 | 236 | 249 | |
| peak 6 | — | — | — | |
| LFP | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
| peak 1 | 160 | 168 | 8 | 448 |
| peak 2 | 180 | 209 | 65 | |
| peak 3 | 197 | 219 | 33 | |
| peak 4 | 198 | 231 | 16 | |
| peak 5 | — | — | — | |
| peak 6 | 222 | 294 | 122 | 240 |

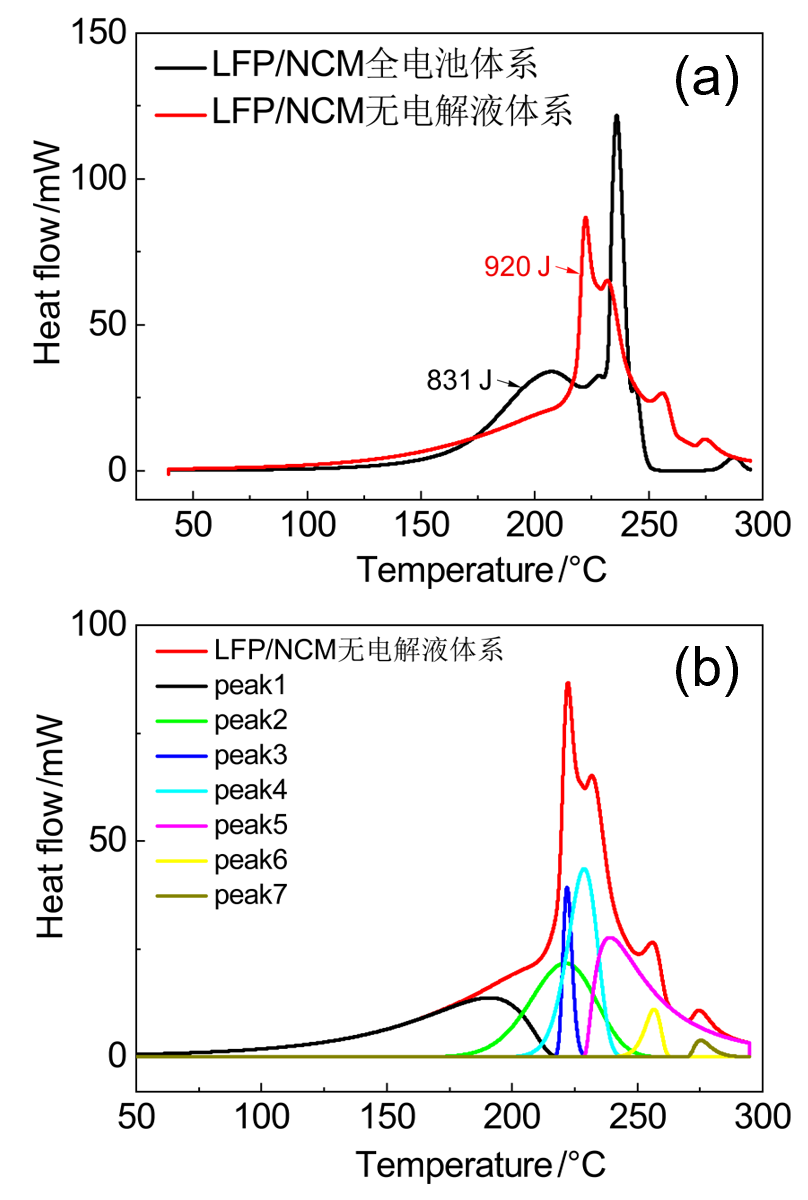
| LFP/NCM无电解液 | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 138 | 194 | 161 | 592 |
| peak 2 | 193 | 221 | 211 | 4057 |
| peak 3 | 218 | 222 | 53 | |
| peak 4 | 215 | 228 | 197 | |
| peak 5 | 229 | 239 | 259 | |
| peak 6 | 248 | 256 | 27 | 125 |
| Peak7 | 270 | 275 | 14 |
| LFP/NCM无电解液 | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于电极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 138 | 194 | 161 | 592 |
| peak 2 | 193 | 221 | 211 | 4057 |
| peak 3 | 218 | 222 | 53 | |
| peak 4 | 215 | 228 | 197 | |
| peak 5 | 229 | 239 | 259 | |
| peak 6 | 248 | 256 | 27 | 125 |
| Peak7 | 270 | 275 | 14 |
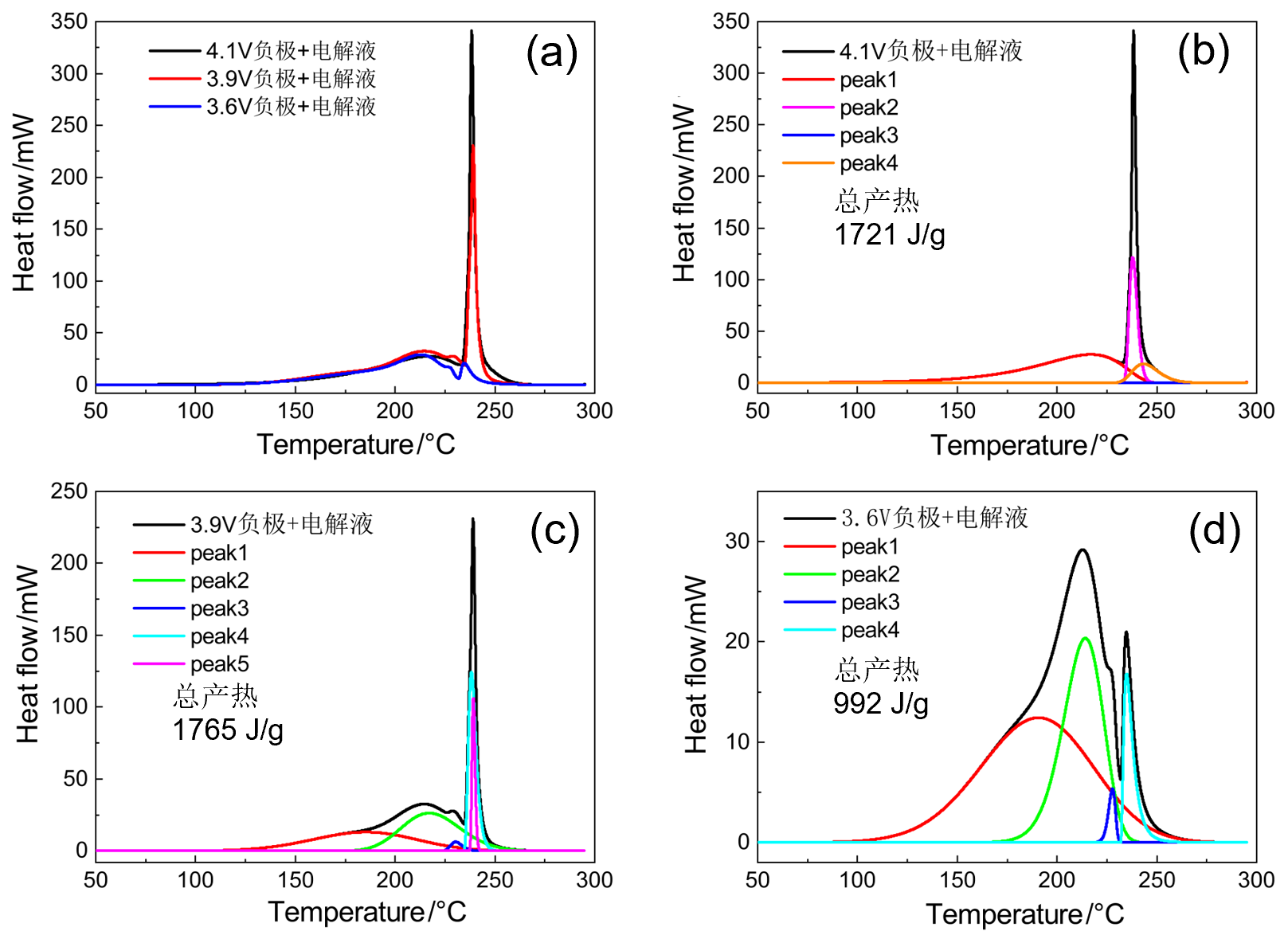
| 4.1V-SOC-90% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 160 | 218 | 845 | 1721 |
| peak 2 | — | — | — | |
| peak 3 | 226 | 229 | 271 | |
| peak 4 | 229 | 232 | 418 | |
| peak 5 | 234 | 243 | 187 | |
| 3.9V-SOC-70% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 130 | 185 | 567 | 1765 |
| peak 2 | 180 | 217 | 622 | |
| peak 3 | 225 | 230 | 30 | |
| peak 4 | 235 | 238 | 413 | |
| peak 5 | 237 | 239 | 133 | |
| 3.6V-SOC-30% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 110 | 190 | 571 | 992 |
| peak 2 | 170 | 214 | 336 | |
| peak 3 | 220 | 227 | 16 | |
| peak 4 | 232 | 234 | 69 | |
| peak 5 | — | — | — |
| 4.1V-SOC-90% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
|---|---|---|---|---|
| peak 1 | 160 | 218 | 845 | 1721 |
| peak 2 | — | — | — | |
| peak 3 | 226 | 229 | 271 | |
| peak 4 | 229 | 232 | 418 | |
| peak 5 | 234 | 243 | 187 | |
| 3.9V-SOC-70% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 130 | 185 | 567 | 1765 |
| peak 2 | 180 | 217 | 622 | |
| peak 3 | 225 | 230 | 30 | |
| peak 4 | 235 | 238 | 413 | |
| peak 5 | 237 | 239 | 133 | |
| 3.6V-SOC-30% | 起始温度/℃ | 峰值温度/℃ | 反应热/J | 基于负极 产热/(J•g-1) |
| peak 1 | 110 | 190 | 571 | 992 |
| peak 2 | 170 | 214 | 336 | |
| peak 3 | 220 | 227 | 16 | |
| peak 4 | 232 | 234 | 69 | |
| peak 5 | — | — | — |
| 样品 | NCM全电池体系(mg) | LFP全电池 体系(mg) | 混合全电池体系(mg) | 负极+电解液体系(mg) |
|---|---|---|---|---|
| 负极极片 | 272 | 272 | 272 | 450 |
| 正极极片 | 507 | 507 | 507 | — |
| 隔膜 | 50 | 50 | 50 | — |
| 电解液 | 150 | 150 | 150 | 240 |
| m(负极)∶m(电解液) | 1.81 | 1.81 | 1.81 | 1.87 |
| 样品 | NCM全电池体系(mg) | LFP全电池 体系(mg) | 混合全电池体系(mg) | 负极+电解液体系(mg) |
|---|---|---|---|---|
| 负极极片 | 272 | 272 | 272 | 450 |
| 正极极片 | 507 | 507 | 507 | — |
| 隔膜 | 50 | 50 | 50 | — |
| 电解液 | 150 | 150 | 150 | 240 |
| m(负极)∶m(电解液) | 1.81 | 1.81 | 1.81 | 1.87 |
| [1] |
Li, M.; Lu, J.; Chen, Z.; Amine, K. Adv. Mater. 2018, 30, 1800561.
doi: 10.1002/adma.v30.33 |
| [2] |
Xu, J.; Cai, X.; Cai, S.; Shao, Y.; Hu, C.; Lu, S.; Ding, S. Energy Environ. Mater. 2023, 6, e12450.
doi: 10.1002/eem2.v6.5 |
| [3] |
Li, J.; Fleetwood, J.; Hawley, W. B.; Kays, W. Chem. Rev. 2022, 122, 903.
doi: 10.1021/acs.chemrev.1c00565 |
| [4] |
Yuan, Z.; Zhang, H.; Hu, S.; Zhang, B.; Zhang, J.; Cui, G. Acta Chim. Sinica 2023, 81, 1064. (in Chinese)
doi: 10.6023/A23030085 |
|
(苑志祥, 张浩, 胡思伽, 张波涛, 张建军, 崔光磊, 化学学报, 2023, 81, 1064.)
doi: 10.6023/A23030085 |
|
| [5] |
Yu, Q.; Nie, Y.; Peng, S.; Miao, Y.; Zhai, C.; Zhang, R.; Han, J.; Zhao, S.; Pecht, M. Appl. Energ. 2023, 349, 121674.
doi: 10.1016/j.apenergy.2023.121674 |
| [6] |
Cai, S.; Zhang, X.; Ji, J. J. Energy Storage 2023, 72, 108750.
doi: 10.1016/j.est.2023.108750 |
| [7] |
Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Energy Storage Mater. 2018, 10, 246.
|
| [8] |
Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. J. Power Sources 2012, 208, 210.
doi: 10.1016/j.jpowsour.2012.02.038 |
| [9] |
Xu, Z.; Zhou, X.; Fu, J.; Li, Q.; Tan, Z.; Fan, X.; Wang, Z.; Tian, B.; Guo, X. Chinese Sci. Bull. 2023, 68, 4501. (in Chinese)
doi: 10.1360/TB-2023-0273 |
|
(徐振恒, 周晓燕, 付佳龙, 李秋桐, 谭则杰, 樊小鹏, 王志明, 田兵, 郭新, 科学通报, 2023, 68, 4501.)
|
|
| [10] |
Zheng, Y.; Che, Y.; Hu, X.; Sui, X.; Stroe, D. I.; Teodorescu, R. Prog. Energ. Combust. 2024, 100, 101120.
doi: 10.1016/j.pecs.2023.101120 |
| [11] |
Khan, M. M.; Alkhedher, M.; Ramadan, M.; Ghazal, M. J. Energy Storage 2023, 73, 108775.
doi: 10.1016/j.est.2023.108775 |
| [12] |
Ma, S.; Jiang, M.; Tao, P.; Song, C.; Wu, J.; Wang, J.; Deng, T.; Shang, W. Prog. Nat. Sci. 2018, 28, 653.
doi: 10.1016/j.pnsc.2018.11.002 |
| [13] |
Ping, P.; Wang, Q.; Huang, P.; Sun, J.; Chen, C. Appl. Energ. 2014, 129, 261.
doi: 10.1016/j.apenergy.2014.04.092 |
| [14] |
Ping, P. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2014. (in Chinese)
|
|
(平平, 博士论文,中国科学技术大学, 合肥, 2014.)
|
|
| [15] |
Huang, P. F. Ph.D. Dissertation, University of Science and Technology of China, Hefei, 2018. (in Chinese)
|
|
(黄沛丰, 博士论文, 中国科学技术大学, 合肥, 2018.)
|
|
| [16] |
Feng, X.; Zheng, S.; Ren, D.; He, X.; Wang, L.; Cui, H.; Liu, X.; Jin, C.; Zhang, F.; Xue, C.; Hsub, H.; Gao, S.; Chen, T.; Li, Y.; Wang, T.; Wang, H.; Li, M.; Ouyang, M. Appl. Energ. 2019, 246, 53.
doi: 10.1016/j.apenergy.2019.04.009 |
| [17] |
Bak, S. M.; Hu, E. Y.; Zhou, Y. N.; Yu, X. Q.; Senanayake, S. D.; Cho, S. J.; Kim, K. B.; Chung, K. Y.; Yang, X. Q.; Nam, K. W. ACS Appl. Mater. Interfaces 2014, 6, 22594.
doi: 10.1021/am506712c |
| [18] |
Li, C.; Wang, H. W.; Han, X. B.; Wang, Y.; Wang, Y.; Zhang, Y. J.; Feng, X. N.; Ouyang, M. G. J. Electrochem. Energy. 2021, 8, 021012.
|
| [19] |
Mao, B. B.; Liu, C. Q.; Yang, K.; Li, S.; Liu, P. J.; Zhang, M. J.; Meng, X. D.; Gao, F.; Duan, Q. L.; Wang, Q. S.; Sun, J. H. Renew. Sust. Energ. Rev. 2021, 139, 110717.
doi: 10.1016/j.rser.2021.110717 |
| [20] |
Wang, Q. S.; Jiang, L. H.; Yu, Y.; Sun, J. H. Nano Energy 2019, 55, 93.
doi: 10.1016/j.nanoen.2018.10.035 |
| [21] |
Zhu, X.; Sun, Z.; Wang, Z.; Wang, H.; Lin, N.; Shan, C. J. Energy Storage 2023, 68, 107768.
doi: 10.1016/j.est.2023.107768 |
| [22] |
Liang, C.; Zhang, W. H.; Wei, Z. S.; Wang, Z. Y.; Wang, Q. S.; Sun, J. H. J. Energy Chem. 2021, 59, 446.
doi: 10.1016/j.jechem.2020.11.024 |
| [23] |
Peng, Y.; Yang, L. Z.; Ju, X. Y.; Liao, B. S.; Ye, K.; Li, L.; Cao, B.; Ni, Y. J. Hazard Mater. 2020, 381, 120916.
doi: 10.1016/j.jhazmat.2019.120916 |
| [24] |
Liang, C.; Jiang, L. H.; Ye, S. L.; Wang, Z. Y.; Wei, Z. S.; Wang, Q. S.; Sun, J. H. J. Energy Chem. 2021, 54, 332.
doi: 10.1016/j.jechem.2020.06.008 |
| [25] |
Duh, Y. S.; Lee, C. Y.; Chen, Y. L.; Kao, C. S. Thermochim. Acta 2016, 642, 88.
doi: 10.1016/j.tca.2016.09.007 |
| [26] |
Jiang, L. H.; Wang, Q. S.; Sun, J. H. J. Hazard Mater. 2018, 351, 260.
doi: 10.1016/j.jhazmat.2018.03.015 |
| [27] |
Duan, J.; Tang, X.; Dai, H. F.; Yang, Y.; Wu, W. Y.; Wei, X. Z.; Huang, Y. H. Electrochem. Energ. Rev. 2020, 3, 1.
doi: 10.1007/s41918-019-00060-4 |
| [1] | 贾洋刚, 陈诗洁, 邵霞, 程婕, 林娜, 方道来, 冒爱琴, 李灿华. 高性能无钴化钙钛矿型高熵氧化物负极材料的制备及储锂性能研究[J]. 化学学报, 2023, 81(5): 486-495. |
| [2] | 张雅岚, 苑志祥, 张浩, 张建军, 崔光磊. 高镍三元高比能固态锂离子电池的研究进展[J]. 化学学报, 2023, 81(12): 1724-1738. |
| [3] | 常婉莹, 谭莹瑛, 吴静怡, 刘英杰, 蔡金海, 赖春艳. 三维结构Li6.28La3Zr2Al0.24O12增强聚氧化乙烯基固态电解质的性能研究[J]. 化学学报, 2023, 81(12): 1708-1715. |
| [4] | 张爽, 杨成飞, 杨玉波, 冯宁宁, 杨刚. 基于废旧锂电池回收制备LixMO (x=0.79, 0.30, 0.08; M=Ni/Co/Mn)材料作为锂-氧气电池正极催化剂的电化学性能研究[J]. 化学学报, 2022, 80(9): 1269-1276. |
| [5] | 陈守潇, 刘君珂, 郑伟琛, 魏国祯, 周尧, 李君涛. 电/离子导体双包覆的LiNi0.8Co0.1Mn0.1O2锂离子电池阴极材料及其电化学性能[J]. 化学学报, 2022, 80(4): 485-493. |
| [6] | 黄擎, 丁瑞, 陈来, 卢赟, 石奇, 张其雨, 聂启军, 苏岳锋, 吴锋. Na2PO3F对LiNi0.83Co0.11Mn0.06O2材料的复合改性及机理分析[J]. 化学学报, 2022, 80(2): 150-158. |
| [7] | 邱凯, 严铭霞, 赵守旺, 安胜利, 王玮, 贾桂霄. Al掺杂的锂离子电池层状正极材料Li(Li0.17Ni0.17Al0.04Fe0.13Mn0.49)O2结构稳定性及氧离子氧化的理论研究[J]. 化学学报, 2021, 79(9): 1146-1153. |
| [8] | 徐平, 张西华, 马恩, 饶富, 刘春伟, 姚沛帆, 孙峙, 王景伟. 退役锂离子电池碳/硫协同选择性提锂技术[J]. 化学学报, 2021, 79(8): 1073-1081. |
| [9] | 李童心, 李东林, 张清波, 高建行, 孔祥泽, 樊小勇, 苟蕾. 大孔高镍LiNi0.8Co0.1Mn0.1O2正极材料的制备及其电化学性能研究[J]. 化学学报, 2021, 79(5): 678-684. |
| [10] | 常智, 乔羽, 杨慧军, 邓瀚, 朱星宇, 何平, 周豪慎. 金属有机框架(MOFs)材料在锂离子电池及锂金属电池电解液中的应用[J]. 化学学报, 2021, 79(2): 139-145. |
| [11] | 刘九鼎, 张宇栋, 刘俊祥, 李金翰, 邱晓光, 程方益. 磷酸锂原位包覆富锂锰基锂离子电池正极材料[J]. 化学学报, 2020, 78(12): 1426-1433. |
| [12] | 任旭强, 李东林, 赵珍珍, 陈光琦, 赵坤, 孔祥泽, 李童心. 铝掺杂及钨酸锂表面包覆双效提升富锂锰基正极材料的循环稳定性[J]. 化学学报, 2020, 78(11): 1268-1274. |
| [13] | 王珊, 樊小勇, 崔宇, 苟蕾, 王新刚, 李东林. 三维多孔集流体改善NiO电极的储锂特性[J]. 化学学报, 2019, 77(6): 551-558. |
| [14] | 王晓钰, 张渝, 马磊, 魏良明. 锂离子电池硅基负极粘结剂发展现状[J]. 化学学报, 2019, 77(1): 24-40. |
| [15] | 邓邦为, 孙大明, 万琦, 王昊, 陈滔, 李璇, 瞿美臻, 彭工厂. 锂离子电池三元正极材料电解液添加剂的研究进展[J]. 化学学报, 2018, 76(4): 259-277. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||