化学学报 ›› 2024, Vol. 82 ›› Issue (3): 314-322.DOI: 10.6023/A23110484 上一篇 下一篇
研究论文
黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪林*( )
)
投稿日期:2023-11-01
发布日期:2024-01-26
基金资助:
Guangzheng Huang, Kunwei Li, Yannan Luo, Qiang Zhang, Yuanlong Pan, Honglin Gao( )
)
Received:2023-11-01
Published:2024-01-26
Contact:
*E-mail: Supported by:文章分享
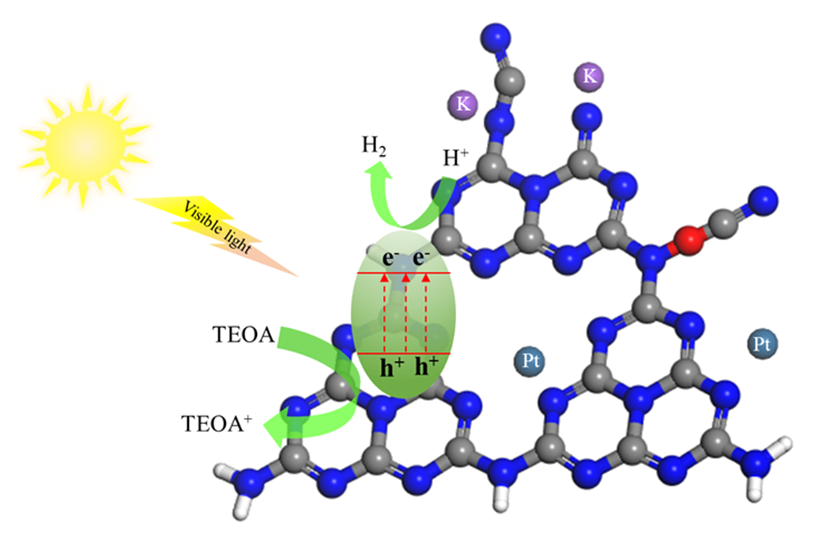
石墨相氮化碳具有可吸收可见光、成本低廉、制备方法简单、稳定性好、无毒等优点, 作为光催化分解水制氢催化剂备受关注, 但其较高的光生载流子复合率、较少的反应活性位点等缺点导致其光催化效率较低. 为提高光生载流子分离效率促进材料表面催化反应进行, 本研究采用简单的氰酸钾水热后处理策略, 通过离子插层剥离和表面偶联反应实现了氮化碳的剥离和表面缺陷构建, 制备了具有K掺杂和表面氰基缺陷的氮化碳纳米片. 后处理提高了材料光催化分解水制氢性能, 其中, CCN-4表现出最优异的光催化还原水制氢性能, 制氢速率为319.5 μmol•g−1•h−1, 是未经后处理氮化碳的6.2倍. 通过一系列表征方法对获得的样品进行详细研究, 发现氰酸钾溶液水热后处理改善了材料的亲水性, 增大了比表面积, 调节了材料的能带结构(导带位负移), 降低了材料与溶液间的电荷传输阻力并提高了光生载流子的分离效率.
黄广峥, 李坤玮, 罗艳楠, 张强, 潘远龙, 高洪林. 水热后处理构建K掺杂和表面缺陷g-C3N4纳米片促进光催化制氢[J]. 化学学报, 2024, 82(3): 314-322.
Guangzheng Huang, Kunwei Li, Yannan Luo, Qiang Zhang, Yuanlong Pan, Honglin Gao. Hydrothermal Treatment for Constructing K Doping and Surface Defects in g-C3N4 Nanosheets Promote Photocatalytic Hydrogen Production[J]. Acta Chimica Sinica, 2024, 82(3): 314-322.









| [1] |
Dai, L.; Chang, D. W.; Baek, J.-B.; Lu, W. Small 2012, 8, 1122.
doi: 10.1002/smll.v8.8 |
| [2] |
An, P.; Zhang, Q.; Yang, Z.; Wu, J.; Zhang, J.; Wang, Y.; Li, Y.; Jiang, G. Acta Chim. Sinica 2022, 80, 1629 (in Chinese).
doi: 10.6023/A22080362 |
|
(安攀, 张庆慧, 杨状, 武佳星, 张佳颖, 王雅君, 李宇明, 姜桂元, 化学学报, 2022, 80, 1629.)
doi: 10.6023/A22080362 |
|
| [3] |
Fujishima, A.; Honda, K. J. n. Nature 1972, 238, 37.
doi: 10.1038/238037a0 |
| [4] |
Wolff, C. M.; Frischmann, P. D.; Schulze, M.; Bohn, B. J.; Wein, R.; Livadas, P.; Carlson, M. T.; Jäckel, F.; Feldmann, J.; Würthner, F.; Stolarczyk, J. K. Nat. Energy 2018, 3, 862.
doi: 10.1038/s41560-018-0229-6 |
| [5] |
Lee, B.-H.; Gong, E.; Kim, M.; Park, S.; Kim, H. R.; Lee, J.; Jung, E.; Lee, C. W.; Bok, J.; Jung, Y.; Kim, Y. S.; Lee, K.-S.; Cho, S.-P.; Jung, J.-W.; Cho, C.-H.; Lebègue, S.; Nam, K. T.; Kim, H.; In, S.-I.; Hyeon, T. Energ. Environ. Sci. 2022, 15, 601.
doi: 10.1039/D1EE01574E |
| [6] |
Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J. M.; Domen, K.; Antonietti, M. Nat. Mater. 2009, 8, 76.
doi: 10.1038/nmat2317 |
| [7] |
Cao, S.; Low, J.; Yu, J.; Jaroniec, M. Adv. Mater. 2015, 27, 2150.
doi: 10.1002/adma.v27.13 |
| [8] |
Xie, Z.; Xue, Z.; Xu, Z.; Li, Q.; Wang, H.; Li, W. Acta Chim. Sinica 2022, 80 1231 (in Chinese).
doi: 10.6023/A22040183 |
|
(解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实, 化学学报, 2022, 80 1231.)
doi: 10.6023/A22040183 |
|
| [9] |
Li, K.; Bao, L.; Cao, S.; Xue, Y.; Yan, S.; Gao, H. ACS Appl. Energy Mater. 2021, 4, 12965.
doi: 10.1021/acsaem.1c02602 |
| [10] |
Wang, S.; Zhao, H.; Zhao, X.; Zhang, J.; Ao, Z.; Dong, P.; He, F.; Wu, H.; Xu, X.; Shi, L.; Zhao, C.; Wang, S.; Sun, H. Chem. Eng. J. 2020, 381, 122593.
doi: 10.1016/j.cej.2019.122593 |
| [11] |
Jang, D.; Lee, S.; Kwon, N. H.; Kim, T.; Park, S.; Jang, K. Y.; Yoon, E.; Choi, S.; Han, J.; Lee, T.-W.; Kim, J.; Hwang, S.-J.; Park, S. Carbon 2023, 208, 290.
doi: 10.1016/j.carbon.2023.03.038 |
| [12] |
Chang, B.; Guo, Y.; Liu, H.; Li, L.; Yang, B. J. Mater. Chem. A 2022, 10, 3134.
doi: 10.1039/D1TA09941H |
| [13] |
Huang, Y.; Mei, F.; Zhang, J.; Dai, K.; Dawson, G. Acta Phys.-Chim. Sin. 2022, 38, 13 (in Chinese).
|
|
(黄悦, 梅飞飞, 张金锋, 代凯, Dawson, G., 物理化学学报, 2022, 38, 13.)
|
|
| [14] |
Wang, X.; Meng, J.; Zhang, X.; Liu, Y.; Ren, M.; Yang, Y.; Guo, Y. Adv. Funct. Mater. 2021, 31, 2010763.
doi: 10.1002/adfm.v31.20 |
| [15] |
Zhao, Z.; Long, Y.; Chen, Y.; Zhang, F.; Ma, J. Chem. Eng. J. 2022, 430, 132682.
doi: 10.1016/j.cej.2021.132682 |
| [16] |
Adekoya, D.; Zhang, S.; Hankel, M. Carbon 2021, 176, 480.
doi: 10.1016/j.carbon.2021.02.050 |
| [17] |
Xie, H.; Li, Z.; Zhu, J.; Li, H.; Yang, Q.; Yang, Y.; Li, C. J. Phys. Chem. Lett. 2022, 13, 11982.
doi: 10.1021/acs.jpclett.2c03250 |
| [18] |
Li, J.; Banis, M. N.; Ren, Z.; Adair, K. R.; Doyle-Davis, K.; Meira, D. M.; Finfrock, Y. Z.; Zhang, L.; Kong, F.; Sham, T.-K.; Li, R.; Luo, J.; Sun, X. Small 2021, 17, 2007245.
doi: 10.1002/smll.v17.11 |
| [19] |
Zhai, B.; Li, H.; Gao, G.; Wang, Y.; Niu, P.; Wang, S.; Li, L. Adv. Funct. Mater. 2022, 32, 2207375.
doi: 10.1002/adfm.v32.47 |
| [20] |
Long, H.; Mo, C.; Mo, Y.; Qiu, C.; Jiang, M. Jiangsu University (Nat. Sci. Ed.) 2020, 41, 712.
|
| [21] |
Lin, B.; Yang, G.; Wang, L. Angew. Chem. Int. Ed. 2019, 58, 4587.
doi: 10.1002/anie.201814360 pmid: 30734453 |
| [22] |
Han, Q.; Wang, B.; Gao, J.; Cheng, Z.; Zhao, Y.; Zhang, Z.; Qu, L. ACS Nano 2016, 10, 2745.
doi: 10.1021/acsnano.5b07831 |
| [23] |
Lu, Y.; Shang, G.; Zhang, H.; Wang, Y.; Tan, Y.; Sun, J.; Liu, G. Chinese J. Inorg. Chem. 2021, 37, 668 (in Chinese).
|
|
(陆杨, 上官莉, 张慧, 王岩, 谭宇烨, 孙建华, 刘光祥, 无机化学学报, 2021, 37, 668.)
|
|
| [24] |
Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Adv. Funct. Mater. 2012, 22, 4763.
doi: 10.1002/adfm.v22.22 |
| [25] |
Wu, P.; Wang, J.; Zhao, J.; Guo, L.; Osterloh, F. E. J. Mater. Chem. A 2014, 2, 20338.
doi: 10.1039/C4TA04100C |
| [26] |
Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. J. Am. Chem. Soc. 2013, 135, 18.
doi: 10.1021/ja308249k |
| [27] |
Dong, C.; Ma, Z.; Qie, R.; Guo, X.; Li, C.; Wang, R.; Shi, Y.; Dai, B.; Jia, X. Appl. Catal. B: Environ. 2017, 217, 629.
doi: 10.1016/j.apcatb.2017.06.028 |
| [28] |
Ruan, X.; Cui, X.; Jia, G.; Wu, J.; Zhao, J.; Singh, D. J.; Liu, Y.; Zhang, H.; Zhang, L.; Zheng, W. Chem. Eng. J. 2022, 428, 132579.
doi: 10.1016/j.cej.2021.132579 |
| [29] |
Zhao, D.; Wang, Y.; Dong, C.-L.; Huang, Y.-C.; Chen, J.; Xue, F.; Shen, S.; Guo, L. Nat. Energy 2021, 6, 388.
doi: 10.1038/s41560-021-00795-9 |
| [30] |
Xu, Y.; He, X.; Zhong, H.; Singh, D. J.; Zhang, L.; Wang, R. Appl. Catal. B: Environ. 2019, 246, 349.
doi: 10.1016/j.apcatb.2019.01.069 |
| [31] |
Murugesan, P.; Moses, J. A.; Anandharamakrishnan, C. J. Mater. Sci. 2019, 54, 12206.
doi: 10.1007/s10853-019-03695-2 |
| [32] |
Wang, C.; Fan, H.; Ren, X.; Ma, J.; Fang, J.; Wang, W. ChemSusChem 2018, 11, 700.
doi: 10.1002/cssc.v11.4 |
| [33] |
Ismael, M. J. Alloy. Compd. 2020, 846, 156446.
doi: 10.1016/j.jallcom.2020.156446 |
| [34] |
Vinu, A.; Ariga, K.; Mori, T.; Nakanishi, T.; Hishita, S.; Golberg, D.; Bando, Y. Adv. Mater. 2005, 17, 1648.
doi: 10.1002/adma.v17:13 |
| [35] |
Lau, V. W.-h.; Yu, V. W.-z.; Ehrat, F.; Botari, T.; Moudrakovski, I.; Simon, T.; Duppel, V.; Medina, E.; Stolarczyk, J. K.; Feldmann, J.; Blum, V.; Lotsch, B. V. Adv. Energy Mater. 2017, 7, 1602251.
doi: 10.1002/aenm.v7.12 |
| [36] |
Wu, S.; Yu, H.; Chen, S.; Quan, X. ACS Catal. 2020, 10, 14380.
doi: 10.1021/acscatal.0c03359 |
| [37] |
Zhang, J.; Chen, X.; Takanabe, K.; Maeda, K.; Domen, K.; Epping, J. D.; Fu, X.; Antonietti, M.; Wang, X. Angew. Chem. Int. Ed. 2010, 49, 441.
doi: 10.1002/anie.v49:2 |
| [38] |
Zhou, Z.; Shen, Y.; Li, Y.; Liu, A.; Liu, S.; Zhang, Y. ACS Nano 2015, 9, 12480.
doi: 10.1021/acsnano.5b05924 |
| [39] |
Yu, Y.; Yan, W.; Wang, X.; Li, P.; Gao, W.; Zou, H.; Wu, S.; Ding, K. Adv. Mater. 2018, 30, 1705060.
doi: 10.1002/adma.v30.9 |
| [40] |
Zhang, M.; Bai, X.; Liu, D.; Wang, J.; Zhu, Y. Appl. Catal. B: Environ. 2015, 164, 77.
doi: 10.1016/j.apcatb.2014.09.020 |
| [41] |
Wang, K.; Fu, J.; Zheng, Y. Appl. Catal. B: Environ. 2019, 254, 270.
doi: 10.1016/j.apcatb.2019.05.002 |
| [42] |
Zhang, G.; Li, G.; Lan, Z.-A.; Lin, L.; Savateev, A.; Heil, T.; Zafeiratos, S.; Wang, X.; Antonietti, M. Angew. Chem. Int. Ed. 2017, 56, 13445.
doi: 10.1002/anie.v56.43 |
| [43] |
Zeng, Z.; Yu, H.; Quan, X.; Chen, S.; Zhang, S. Appl. Catal. B: Environ. 2018, 227, 153.
doi: 10.1016/j.apcatb.2018.01.023 |
| [44] |
Jin, Z.; Jiang, X.; Zhang, Q.; Huang, S.; Zhang, L.; Huang, L.; He, T.; Zhang, H.; Ohno, T.; Ruan, S.; Zeng, Y.-J. Commun. Mater. 2020, 1, 90.
doi: 10.1038/s43246-020-00093-z |
| [45] |
Li, Y.; Xue, Y.; Gao, X.; Wang, L.; Liu, X.; Wang, Z.; Shen, S. Adv. Funct. Mater. 2023, n/a, 2312634.
|
| [46] |
Liu, C.; Zhang, Y.; Dong, F.; Reshak, A. H.; Ye, L.; Pinna, N.; Zeng, C.; Zhang, T.; Huang, H. Appl. Catal. B: Environ. 2017, 203, 465.
doi: 10.1016/j.apcatb.2016.10.002 |
| [47] |
Guo, Y.; Bae, J.; Fang, Z.; Li, P.; Zhao, F.; Yu, G. Chem. Rev. 2020, 120, 7642.
doi: 10.1021/acs.chemrev.0c00345 |
| [1] | 张东彬, 袁欣然, 辛亚男, 刘天豪, 韩慧果, 杜光超, 滕艾均. 磷酸氧钒钠纳米片的可控制备研究[J]. 化学学报, 2024, 82(3): 274-280. |
| [2] | 陈健强, 朱钢国, 吴劼. 镍催化氮杂环丙烷的开环偶联反应研究[J]. 化学学报, 2024, 82(2): 190-212. |
| [3] | 吴宇晗, 张栋栋, 尹宏宇, 陈正男, 赵文, 匙玉华. “双碳”目标下Janus In2S2X光催化还原CO2的密度泛函理论研究[J]. 化学学报, 2023, 81(9): 1148-1156. |
| [4] | 刘嘉文, 林玮璜, 王惟嘉, 郭学益, 杨英. Cu1.94S-SnS纳米异质结的合成及其光催化降解研究[J]. 化学学报, 2023, 81(7): 725-734. |
| [5] | 何明慧, 叶子秋, 林桂庆, 尹晟, 黄心翊, 周旭, 尹颖, 桂波, 汪成. 卟啉基共价有机框架的光催化研究进展★[J]. 化学学报, 2023, 81(7): 784-792. |
| [6] | 李飞, 丁汇丽, 李超忠. 基于氟仿衍生的三氟甲基硼络合物参与的烯烃氢三氟甲基化反应[J]. 化学学报, 2023, 81(6): 577-581. |
| [7] | 刘坜, 郑刚, 范国强, 杜洪光, 谭嘉靖. 4-酰基/氨基羰基/烷氧羰基取代汉斯酯参与的有机反应研究进展[J]. 化学学报, 2023, 81(6): 657-668. |
| [8] | 徐袁利, 潘辉, 杨义, 左智伟. 连续流条件下蒽-铈协同催化的苄位碳氢键选择性氧化反应★[J]. 化学学报, 2023, 81(5): 435-440. |
| [9] | 齐学平, 王飞, 张健. 后合成法构筑钛基金属有机框架及其应用[J]. 化学学报, 2023, 81(5): 548-558. |
| [10] | 蒋江民, 郑欣冉, 孟雅婷, 贺文杰, 陈亚鑫, 庄全超, 袁加仁, 鞠治成, 张校刚. 氟氮共掺杂多孔碳纳米片的制备及其储钾性能研究[J]. 化学学报, 2023, 81(4): 319-327. |
| [11] | 陈健强, 朱钢国, 吴劼. 草酸酯类化合物在自由基脱羟基化反应中的研究进展[J]. 化学学报, 2023, 81(11): 1609-1623. |
| [12] | 杨春晖, 陈景超, 李新汉, 孟丽, 王凯民, 孙蔚青, 樊保敏. 可见光催化的硅烷二氟烯丙基化反应[J]. 化学学报, 2023, 81(1): 1-5. |
| [13] | 解众舒, 薛中鑫, 许子文, 李倩, 王洪宇, 李维实. 石墨相氮化碳的共轭交联修饰及其对可见光催化产氢性能的影响[J]. 化学学报, 2022, 80(9): 1231-1237. |
| [14] | 祁育, 章福祥. 太阳能光催化分解水制氢※[J]. 化学学报, 2022, 80(6): 827-838. |
| [15] | 舒恒, 包义德日根, 那永. CdS基纳米管光催化氧化5-羟甲基糠醛选择性生成2,5-呋喃二甲醛[J]. 化学学报, 2022, 80(5): 607-613. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||