化学学报 ›› 2024, Vol. 82 ›› Issue (3): 367-376.DOI: 10.6023/A23100448 上一篇
综述
投稿日期:2023-10-13
发布日期:2024-02-02
作者简介: |
王博, 兰州大学化学化工学院在读硕士生, 2020年6月于西安交通大学化学工程与工艺专业获得学士学位, 随后加入兰州大学化学化工学院肖建喜教授课题组, 主要研究方向为多肽探针的设计及其在肿瘤检测方面的应用. |
 |
蔡向东, 博士, 分别于2015年和2021年在兰州大学化学化工学院化学专业获得学士学位和博士学位, 随后在兰州大学生命科学学院从事博士后研究工作. 主要研究方向为基于多肽化学的肿瘤诊断和治疗. |
 |
肖建喜, 教授, 博士生导师. 2003年获武汉大学化学学士学位, 2009年获美国新泽西州罗格斯大学(Rutgers-New Brunswick)化学博士学位. 2009~2011年在罗格斯大学从事博士后研究工作. 2012年获聘为兰州大学教授. 主要研究领域包括生物分析化学, 生物医用材料和蛋白质工程与产业化应用等. 已发表多篇SCI期刊论文, 申请发明专利40余项. |
基金资助:
Bo Wanga,b, Xiangdong Caia,b( ), Jianxi Xiaoa,b(
), Jianxi Xiaoa,b( )
)
Received:2023-10-13
Published:2024-02-02
Contact:
*E-mail: Supported by:文章分享
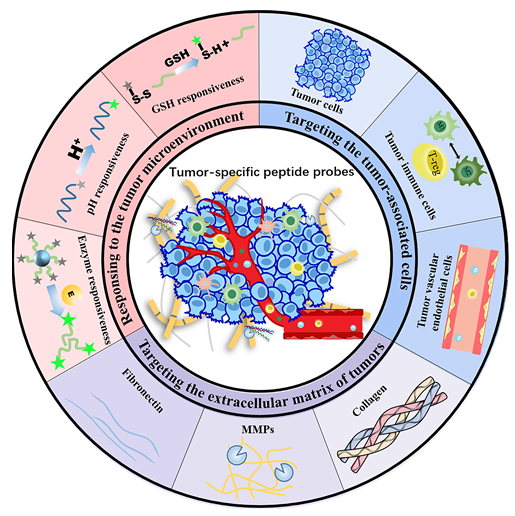
肿瘤是由细胞、细胞外基质及微环境等多因素组成的复杂系统, 不同因素在肿瘤的发生和发展中发挥关键作用. 肿瘤细胞、细胞外基质及微环境的特异性分析对于肿瘤的精准诊断和靶向治疗至关重要. 多肽探针具有特异性高、生物相容性好、组织穿透能力强和易于制备等优点, 已被广泛用于肿瘤的特异性成像研究. 本文综述了多肽探针对肿瘤细胞、免疫细胞等细胞靶点的特异性成像; 并介绍了以胶原蛋白、纤维蛋白等外基质蛋白为靶点的肿瘤特异性多肽探针的成像研究. 本文还总结了对肿瘤微环境中弱酸性、高酶活性等因素响应的肿瘤特异性多肽探针及其生物成像应用. 最后, 本文总结并讨论了肿瘤特异性多肽探针所面临的挑战与机遇, 展望了其在肿瘤精准诊断和个性化治疗领域的前景.
王博, 蔡向东, 肖建喜. 肿瘤特异性多肽探针及其在生物成像中的应用[J]. 化学学报, 2024, 82(3): 367-376.
Bo Wang, Xiangdong Cai, Jianxi Xiao. Tumor-specific Peptide Probes and the Applications in Bioimaging[J]. Acta Chimica Sinica, 2024, 82(3): 367-376.
| Cells | Targets | Sequences | Applications | Ref. |
|---|---|---|---|---|
| Tumor cells | HER2 | LTVSPWY | SKBR3 cells | [ |
| EGFR | YHWYGYTPQNVI | SMMC-7721 and K562 cells | [ | |
| VEGFR-3 | CSDSWHYWC | HT29 cells | [ | |
| Tf-R | CRTIGPSVC | glioblastoma xenograft-bearing mice | [ | |
| FRα | MHTAPGWGYRLS | SKOV3 cells | [ | |
| CD44 | WHPWSYLWTQQA | SGC-7901, BGC-823 and MKN-28 cells | [ | |
| CD133 | LQNAPRS | CT-26, MA-782, UMR106 and SW480 cells | [ | |
| Immune cells | CD206 | CSPGAKVRC | B16F10 tumor-bearing mice | [ |
| Nrp1 | CGNKRTRGC | B16BL/6 tumor-bearing mice | [ | |
| Endothelial cells | Nucleolin | KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK | — | [ |
| αvβ3 and αvβ5 integrin | CDCRGDCFC | MDA-MB-435 tumor-bearing mice | [ | |
| Plectin-1 and integrin | KTLLPTPKRGDFKK | Panc1 tumor-bearing mice | [ |
| Cells | Targets | Sequences | Applications | Ref. |
|---|---|---|---|---|
| Tumor cells | HER2 | LTVSPWY | SKBR3 cells | [ |
| EGFR | YHWYGYTPQNVI | SMMC-7721 and K562 cells | [ | |
| VEGFR-3 | CSDSWHYWC | HT29 cells | [ | |
| Tf-R | CRTIGPSVC | glioblastoma xenograft-bearing mice | [ | |
| FRα | MHTAPGWGYRLS | SKOV3 cells | [ | |
| CD44 | WHPWSYLWTQQA | SGC-7901, BGC-823 and MKN-28 cells | [ | |
| CD133 | LQNAPRS | CT-26, MA-782, UMR106 and SW480 cells | [ | |
| Immune cells | CD206 | CSPGAKVRC | B16F10 tumor-bearing mice | [ |
| Nrp1 | CGNKRTRGC | B16BL/6 tumor-bearing mice | [ | |
| Endothelial cells | Nucleolin | KDEPQRRSARLSAKPAPPKPEPKPKKAPAKK | — | [ |
| αvβ3 and αvβ5 integrin | CDCRGDCFC | MDA-MB-435 tumor-bearing mice | [ | |
| Plectin-1 and integrin | KTLLPTPKRGDFKK | Panc1 tumor-bearing mice | [ |
| Extracellular matrix | Sequences | Applications | Ref. |
|---|---|---|---|
| Pathological collagen | AF647-(GPO)9 | 3D cells of MDA-MB-231 and MCF-7 | [ |
| CF-G3(GPO)4NBGPO(GPO)4 | PC-3 tumor-bearing mice | [ | |
| ROX-Ahx-(GPO)7-D6-NH2 | Rectal, lung, cervical and breast cancer tissue | [ | |
| FAM-(GPP)8 | Leiomyoma, gastric and rectal cancer tissue | [ | |
| FAM-(GOP)10 | Gastric, lung, colorectal and breast cancers tissue | [ | |
| MMP-2、MMP-9 | CTTHWGFTLC | B16F10 and MDA-MB-435 tumor-bearing mice | [ |
| MMP-14 | HWKHLHNTKTFL | MDA-MB-435 xenograft model mice | [ |
| HSVEDVS | HT1080 and A549 tumor-bearing mice | [ | |
| MMP-16 | ASTSMLRTILGP | HT-1080 cells and imaging of the tumor model mice | [ |
| Fibrin | GGGGY-dGlu-C-Hyp-3ClY-GLCYIQG-NH2 | BT-20 tumor-bearing mice | [ |
| Tenascin | FHKHKSPALSPVGGG | U251 and HCT116 cells; lung cancer tissue | [ |
| EDB-FN | CTVRTSADC | PC3 tumor-bearing mice | [ |
| Fibronectin-fibronectin | CREKA | 4T1 tumor-bearing mice | [ |
| Extracellular matrix | Sequences | Applications | Ref. |
|---|---|---|---|
| Pathological collagen | AF647-(GPO)9 | 3D cells of MDA-MB-231 and MCF-7 | [ |
| CF-G3(GPO)4NBGPO(GPO)4 | PC-3 tumor-bearing mice | [ | |
| ROX-Ahx-(GPO)7-D6-NH2 | Rectal, lung, cervical and breast cancer tissue | [ | |
| FAM-(GPP)8 | Leiomyoma, gastric and rectal cancer tissue | [ | |
| FAM-(GOP)10 | Gastric, lung, colorectal and breast cancers tissue | [ | |
| MMP-2、MMP-9 | CTTHWGFTLC | B16F10 and MDA-MB-435 tumor-bearing mice | [ |
| MMP-14 | HWKHLHNTKTFL | MDA-MB-435 xenograft model mice | [ |
| HSVEDVS | HT1080 and A549 tumor-bearing mice | [ | |
| MMP-16 | ASTSMLRTILGP | HT-1080 cells and imaging of the tumor model mice | [ |
| Fibrin | GGGGY-dGlu-C-Hyp-3ClY-GLCYIQG-NH2 | BT-20 tumor-bearing mice | [ |
| Tenascin | FHKHKSPALSPVGGG | U251 and HCT116 cells; lung cancer tissue | [ |
| EDB-FN | CTVRTSADC | PC3 tumor-bearing mice | [ |
| Fibronectin-fibronectin | CREKA | 4T1 tumor-bearing mice | [ |
| Responsive types | Sequences | Applications | Ref. |
|---|---|---|---|
| pH | AAEQNPIYWARYADWLFTTPLLLLDLALLVDADEGTCG | M4A4 and NM2C5 cells tumor-bearing mice | [ |
| ACEQNPIYWARYANWLFTTPLLLLNLALLVDADEGTG | Breast cancer | [ | |
| ACDDQNPWRAYLDLLFPTDTLLLDLLW | MDA-MB-231, 4T1, A549 and LLC tumor- bearing mice | [ | |
| H6 | Breast cancer tumor-bearing mice | [ | |
| MMP-2 | PLGLAG | HT-1080 cells and HT-1080 tumor-bearing mice | [ |
| GPLGVRGK | HT29 tumor-bearing mice | [ | |
| PLGVR | HT-1080 cells and HT-1080 tumor-bearing mice | [ | |
| GPLGIAGQ | MCF-7, MDR and L02 cells and tumor- bearing mice | [ | |
| MMP-14 | RS-Cit-G-HPhe-YLY | U87 cells and U87 tumor-bearing | [ |
| Cathepsin B | GFLG | HeLa cells and H640 tumor-bearing | [ |
| Cathepsin S | PMGLP | HPAC tumor-bearing | [ |
| GSH | e4(Ce4)-S-S-YHWYGYTPQNVI | Triple negative breast cancer tumor-bearing | [ |
| Multi- responsiveness | MMP-2: GPLGVRG, MMP-7: VPLSLTMG | HepG2 and HL7702 | [ |
| pH: R9, MMP-2: PLGLAG | Glioma distribution in mice | [ | |
| pH: HLAH-LAHHLAHLAH, MMP-2: GPLGIAGQC | U87 cells | [112] |
| Responsive types | Sequences | Applications | Ref. |
|---|---|---|---|
| pH | AAEQNPIYWARYADWLFTTPLLLLDLALLVDADEGTCG | M4A4 and NM2C5 cells tumor-bearing mice | [ |
| ACEQNPIYWARYANWLFTTPLLLLNLALLVDADEGTG | Breast cancer | [ | |
| ACDDQNPWRAYLDLLFPTDTLLLDLLW | MDA-MB-231, 4T1, A549 and LLC tumor- bearing mice | [ | |
| H6 | Breast cancer tumor-bearing mice | [ | |
| MMP-2 | PLGLAG | HT-1080 cells and HT-1080 tumor-bearing mice | [ |
| GPLGVRGK | HT29 tumor-bearing mice | [ | |
| PLGVR | HT-1080 cells and HT-1080 tumor-bearing mice | [ | |
| GPLGIAGQ | MCF-7, MDR and L02 cells and tumor- bearing mice | [ | |
| MMP-14 | RS-Cit-G-HPhe-YLY | U87 cells and U87 tumor-bearing | [ |
| Cathepsin B | GFLG | HeLa cells and H640 tumor-bearing | [ |
| Cathepsin S | PMGLP | HPAC tumor-bearing | [ |
| GSH | e4(Ce4)-S-S-YHWYGYTPQNVI | Triple negative breast cancer tumor-bearing | [ |
| Multi- responsiveness | MMP-2: GPLGVRG, MMP-7: VPLSLTMG | HepG2 and HL7702 | [ |
| pH: R9, MMP-2: PLGLAG | Glioma distribution in mice | [ | |
| pH: HLAH-LAHHLAHLAH, MMP-2: GPLGIAGQC | U87 cells | [112] |
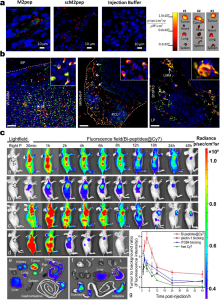

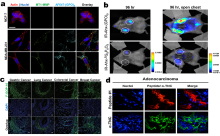

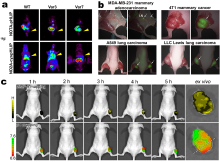

| Name | Indication |
|---|---|
| 64Cu DOTA-TATE Detectnet® | neuroendocrine tumors |
| 68Ga PSMA-11 | prostate cancer |
| Piflufolastat F18 injection Pylarify® | prostate cancer |
| Name | Indication |
|---|---|
| 64Cu DOTA-TATE Detectnet® | neuroendocrine tumors |
| 68Ga PSMA-11 | prostate cancer |
| Piflufolastat F18 injection Pylarify® | prostate cancer |
| [1] |
Henke, E.; Nandigama, R.; Ergün, S. Front. Mol. Biosci. 2019, 6, 160.
doi: 10.3389/fmolb.2019.00160 pmid: 32118030 |
| [2] |
Bejarano, L.; Jordāo, M. J. C.; Joyce, J. A. Cancer Discov. 2021, 11, 933.
doi: 10.1158/2159-8290.CD-20-1808 pmid: 33811125 |
| [3] |
Sa, P.; Sahoo, S. K.; Dilnawaz, F. Curr. Med. Chem. 2023, 30, 3335.
doi: 10.2174/0929867329666220922111336 |
| [4] |
Monteiro, A. C.; Lepique, A. P.; Bonamino, M.; Fuertes, M. B. Front. Immunol. 2023, 14, 1141084.
doi: 10.3389/fimmu.2023.1141084 |
| [5] |
Peng, S. J.; Xiao, F. F.; Chen, M. W.; Gao, H. L. Adv. Sci. 2022, 9, 2103836.
doi: 10.1002/advs.v9.1 |
| [6] |
Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F. X. C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Cancer Med. 2023, 12, 11149.
doi: 10.1002/cam4.v12.10 |
| [7] |
Binnewies, M.; Roberts, E. W.; Krummel, M. F. Nat. Med. 2018, 24, 541.
doi: 10.1038/s41591-018-0014-x pmid: 29686425 |
| [8] |
de Visser, K. E.; Joyce, J. A. Cancer Cell 2023, 41, 374.
doi: 10.1016/j.ccell.2023.02.016 pmid: 36917948 |
| [9] |
Junttila, M. R.; de Sauvage, F. J. Nature 2013, 501, 346.
doi: 10.1038/nature12626 |
| [10] |
Kashyap, B. K.; Singh, V. V.; Solanki, M. K.; Kumar, A.; Ruokolainen, J.; Kesari, K. K. ACS Omega 2023, 8, 14290.
doi: 10.1021/acsomega.2c07840 |
| [11] |
Nirmala, M. J.; Kizhuveetil, U.; Johnson, A.; Balaji, G.; Nagarajan, R.; Muthuvijayan, V. RSC Adv. 2023, 13, 8606.
doi: 10.1039/d2ra07863e pmid: 36926304 |
| [12] |
Kondo, E.; Iioka, H.; Saito, K. Cancer Sci. 2021, 112, 2118.
doi: 10.1111/cas.v112.6 |
| [13] |
Shim, H. Biomolecules 2020, 10, 360.
doi: 10.3390/biom10030360 |
| [14] |
Yan, J.; Gao, T.; Lu, Z.; Yin, J.; Zhang, Y.; Pei, R. ACS Appl. Mater. Interfaces 2021, 13, 27749.
doi: 10.1021/acsami.1c06818 |
| [15] |
Mo, T.; Liu, X.; Luo, Y.; Zhong, L.; Zhang, Z.; Li, T.; Gan, L.; Liu, X.; Li, L.; Wang, H.; Sun, X.; Fan, D.; Qian, Z.; Wu, P.; Chen, X. Cancer Sci. 2022, 113, 7.
doi: 10.1111/cas.v113.1 |
| [16] |
Ma, T.; Hou, Y.; Zeng, J.; Liu, C.; Zhang, P.; Jing, L.; Shangguan, D.; Gao, M. J. Am. Chem. Soc. 2018, 140, 211.
doi: 10.1021/jacs.7b08900 |
| [17] |
Lin, Y.; Wang, S.; Zhang, Y.; Gao, J.; Hong, L.; Wang, X.; Wu, W.; Jiang, X. J. Mater. Chem. B 2015, 3, 5702.
doi: 10.1039/C5TB00593K |
| [18] |
Davis, K. M.; Ryan, J. L.; Aaron, V. D.; Sims, J. B. Semin. Ultrasound CT MR 2020, 41, 521.
doi: 10.1053/j.sult.2020.08.006 |
| [19] |
Lu, Z. R.; Laney, V.; Li, Y. Acc. Chem. Res. 2022, 55, 2833.
doi: 10.1021/acs.accounts.2c00346 |
| [20] |
Nicolescu, C.; Schilb, A.; Kim, J.; Sun, D.; Hall, R.; Gao, S.; Gilmore, H.; Schiemann, W. P.; Lu, Z. R. Chem. Biomed. Eng. 2023, 1, 461.
doi: 10.1021/cbmi.3c00011 pmid: 37655165 |
| [21] |
Zhu, S.; Tian, R.; Antaris, A. L.; Chen, X.; Dai, H. Adv. Mater. 2019, 31, 1900321.
doi: 10.1002/adma.v31.24 |
| [22] |
Zhao, D.; Cao, J.; Zhang, L.; Zhang, S.; Wu, S. Biosensors (Basel) 2022, 12, 342.
|
| [23] |
Merkes, J. M.; Kiessling, F.; Banala, S. Curr. Med. Chem. 2022, 29, 6008.
doi: 10.2174/0929867329666220208093735 |
| [24] |
Santis, E. D.; Ryadnov, M. G. Chem. Soc. Rev. 2015, 44, 8288.
doi: 10.1039/C5CS00470E |
| [25] |
Lu, L.; Zhang, Q.; Wang, Z.; Gao, L.; Shen, J. Curr. Med. Chem. 2021, 28, 6411.
doi: 10.2174/0929867327666201022122131 |
| [26] |
Zhang, C.; Wu, W.; Li, R. Q.; Qiu, W. X.; Zhuang, Z. N.; Cheng, S. X.; Zhang, X. Z. Adv. Funct. Mater. 2018, 28, 1804492.
doi: 10.1002/adfm.v28.50 |
| [27] |
Shadidi, M.; Sioud, M. FASEB J. 2023, 17, 256.
doi: 10.1096/fsb2.v17.2 |
| [28] |
Xing, L.; Xu, Y.; Sun, K.; Wang, H.; Zhang, F.; Zhou, Z.; Zhang, J.; Zhang, F.; Caliskan, B.; Qiu, Z.; Wang, M. Sci. Rep. 2018, 8, 8426.
doi: 10.1038/s41598-018-26683-z |
| [29] |
Qin, X.; Wan, Y.; Li, M.; Xue, X. C.; Wu, S. Z.; Zhang, C.; You, Y. J.; Wang, W. H.; Jiang, C. L.; Liu, Y.; Zhu, W. H.; Ran, Y. G.; Zhang, Z.; Han, W.; Zhang, Y. Q. J. Biochem. 2007, 142, 79.
|
| [30] |
Goldbloom-Helzner, L.; Hao, D.; Wang, A. Int. J. Mol. Sci. 2019, 20, 4072.
doi: 10.3390/ijms20174072 |
| [31] |
Gong, F.; Yang, N.; Wang, X.; Zhao, Q.; Chen, Q.; Liu, Z.; Cheng, L. Nano Today 2020, 32, 100851.
doi: 10.1016/j.nantod.2020.100851 |
| [32] |
Trac, N. T.; Chung, E. J. Bioact. Mater. 2020, 5, 92.
|
| [33] |
David, A. Adv. Drug Deliver. Rev. 2017, 119, 120.
doi: 10.1016/j.addr.2017.05.006 |
| [34] |
Chen, Y.; Liu, G.; Guo, L.; Wang, H.; Fu, Y.; Luo, Y. Int. J. Cancer 2015, 136, 182.
doi: 10.1002/ijc.v136.1 |
| [35] |
Ries, J.; Vairaktaris, E.; Agaimy, A.; Bechtold, M.; Gorecki, P.; Neukam, F. W.; Nkenke, E. Oncol. Rep. 2013, 30, 1149.
doi: 10.3892/or.2013.2545 |
| [36] |
Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A. L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; Stockmann, C.; Combe, P.; Berger, A.; Zinzindohoue, F.; Yagita, H.; Tartour, E.; Taieb, J.; Terme, M. J. Exp. Med. 2015, 212, 139.
doi: 10.1084/jem.20140559 |
| [37] |
Ellis, L. M.; Hicklin, D. J. Nat. Rev. Cancer 2008, 8, 579.
doi: 10.1038/nrc2403 pmid: 18596824 |
| [38] |
Jordan, N. V.; Bardia, A.; Wittner, B. S.; Benes, C.; Ligorio, M.; Zheng, Y.; Yu, M.; Sundaresan, T. K.; Licausi, J. A.; Desai, R.; O’Keefe, R. M.; Ebright, R. Y.; Boukhali, M.; Sil, S.; Onozato, M. L.; Iafrate, A. J.; Kapur, R.; Sgroi, D.; Ting, D. T.; Toner, M.; Ramaswamy, S.; Haas, W.; Maheswaran, S.; Haber, D. A. Nature 2016, 537, 102.
doi: 10.1038/nature19328 |
| [39] |
Cao, R.; Li, R.; Shi, H.; Liu, H.; Cheng, Z. Mol. Pharmaceutics 2023, 20, 1394.
doi: 10.1021/acs.molpharmaceut.2c00964 |
| [40] |
Huang, W.; He, Z.; Cai, X.; Zhang, J.; Li, W.; Wang, K.; Zhang, S. Biosensors 2022, 12, 729.
doi: 10.3390/bios12090729 |
| [41] |
Fiacco, S. V.; Kelderhouse, L. E.; Hardy, A.; Peleg, Y.; Hu, B.; Ornelas, A.; Yang, P.; Gammon, S. T.; Howell, S. M.; Wang, P.; Takahashi, T. T.; Millward, S. W.; Roberts, R. W. ChemBioChem 2016, 17, 1643.
doi: 10.1002/cbic.v17.17 |
| [42] |
Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. FASEB J. 2005, 19, 1978.
doi: 10.1096/fsb2.v19.14 |
| [43] |
Huang, W.; Wang, L.; Zhang, H.; Han, Z.; Gu, Y. Sensor. Actuat. B-Chem. 2023, 393, 134102.
doi: 10.1016/j.snb.2023.134102 |
| [44] |
Xu, Y.; Zhao, Y.; Zhang, Y. J.; Cui, Z. F.; Wang, L. H.; Fan, C. H.; Gao, J. M.; Sun, Y. H. Acta Chim. Sinica 2018, 76, 393 (in Chinese).
doi: 10.6023/A18010039 |
|
(徐毅, 赵彦, 张叶俊, 崔之芬, 王丽华, 樊春海, 高基民, 孙艳红, 化学学报, 2018, 76, 393.)
doi: 10.6023/A18010039 |
|
| [45] |
Hao, Y.; Luo, J.; Wang, Y.; Li, Z.; Wang, X.; Yan, F. Biomaterials 2023, 293, 121974.
doi: 10.1016/j.biomaterials.2022.121974 |
| [46] |
Xie, W.; Yang, P.; Zeng, X.; Wang, H.; Cai, H.; Cai, J. Chin. Sci. Bull. 2010, 55, 2390.
doi: 10.1007/s11434-010-3284-3 |
| [47] |
Staquicini, F. I.; Ozawa, M. G.; Moya, C. A.; Driessen, W. H. P.; Barbu, E. M.; Nishimori, H.; Soghomonyan, S.; Flores, L. G.; Liang, X.; Paolillo, V.; Alauddin, M. M.; Basilion, J. P.; Furnari, F. B.; Bogler, O.; Lang, F. F.; Aldape, K. D.; Fuller, G. N.; Höök, M.; Gelovani, J. G.; Sidman, R. L.; Cavenee, W. K.; Pasqualini, R.; Arap, W. J. Clin. Invest. 2011, 121, 161.
doi: 10.1172/JCI44798 pmid: 21183793 |
| [48] |
Su, C. Y.; Huang, G. C.; Chang, Y. C.; Chen, Y. J.; Fang, H. W. In Vivo 2021, 35, 1545.
doi: 10.21873/invivo.12408 pmid: 33910833 |
| [49] |
Hou, Y.; Zou, Q.; Ge, R.; Shen, F.; Wang, Y. Cell Res. 2012, 22, 259.
doi: 10.1038/cr.2011.139 |
| [50] |
Zhang, D.; Jia, H.; Wang, Y.; Li, W. M.; Hou, Y. C.; Yin, S. W.; Wang, T. D.; He, S. X.; Lu, S. Y. Biotechnol. Lett. 2015, 37, 2311.
doi: 10.1007/s10529-015-1896-z pmid: 26140900 |
| [51] |
Wang, Z.; Zhao, C.; Li, Y.; Wang, J.; Hou, D.; Wang, L.; Wang, Y.; Wang, X.; Liu, X.; Wang, H.; Xu, W. Adv. Mater. 2023, 35, 2210732.
doi: 10.1002/adma.v35.35 |
| [52] |
Sun, J.; Zhang, C.; Liu, G.; Liu, H.; Zhou, C.; Lu, Y.; Zhou, C.; Yuan, L.; Li, X. Clin. Exp. Metastasis 2012, 29, 185.
doi: 10.1007/s10585-011-9440-6 |
| [53] |
Wang, Y.; Yan, K.; Wang, J.; Lin, J.; Bi, J. Front. Oncol. 2021, 11, 609334.
doi: 10.3389/fonc.2021.609334 |
| [54] |
Cieslewicz, M.; Tang, J.; Yu, J. L.; Cao, H.; Zavaljevski, M.; Motoyama, K.; Lieber, A.; Raines, E. W.; Pun, S. H. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 15919.
doi: 10.1073/pnas.1312197110 |
| [55] |
Huang, M.; Wang, R.; Li, M.; Cai, H.; Tian, R. Pharmaceutics 2022, 14, 2511.
doi: 10.3390/pharmaceutics14112511 |
| [56] |
Scodeller, P.; Simón-Gracia, L.; Kopanchuk, S.; Tobi, A.; Kilk, K.; Säälik, P.; Kurm, K.; Squadrito, M. L.; Kotamraju, V. R.; Rinken, A.; De Palma, M.; Ruoslahti, E.; Teesalu, T. Sci. Rep. 2017, 7, 14655.
doi: 10.1038/s41598-017-14709-x pmid: 29116108 |
| [57] |
Ou, W.; Thapa, R. K.; Jiang, L.; Soe, Z. C.; Gautam, M.; Chang, J. H.; Jeong, J. H.; Ku, S. K.; Choi, H. G.; Yong, C. S.; Kim, J. O. J. Control. Release 2018, 281, 84.
doi: 10.1016/j.jconrel.2018.05.018 |
| [58] |
Curiel, T. J.; Morris, C.; Brumlik, M.; Landry, S. J.; Finstad, K.; Nelson, A.; Joshi, V.; Hawkins, C.; Alarez, X.; Lackner, A.; Mohamadzadeh, M. J. Immunol. 2004, 172, 7425.
pmid: 15187120 |
| [59] |
Ihanus, E.; Uotila, L. M.; Toivanen, A.; Varis, M.; Gahmberg, C. G. Blood 2007, 109, 802.
doi: 10.1182/blood-2006-04-014878 |
| [60] |
McDonald, D. M.; Baluk, P. Cancer Res. 2002, 62, 5381.
pmid: 12235011 |
| [61] |
Maishi, N.; Hida, K. Cancer Sci. 2017, 108, 1921.
doi: 10.1111/cas.2017.108.issue-10 |
| [62] |
Christian, S.; Pilch, J.; Akerman, M. E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. J. Cell Biol. 2003, 163, 871.
doi: 10.1083/jcb.200304132 pmid: 14638862 |
| [63] |
Zheng, Y.; Gao, S.; Ying, J. Y. Adv. Mater. 2007, 19, 376.
doi: 10.1002/adma.v19:3 |
| [64] |
Pasqualini, R.; Koivunen, E.; Ruoslahti, E. Nat. Biotechnol. 1997, 15, 542.
pmid: 9181576 |
| [65] |
Zhu, H.; Li, N.; Lin, X.; Hong, Y.; Yang, Z. Acta Chim. Sinica 2014, 72, 427 (in Chinese).
doi: 10.6023/A13121248 |
|
(朱华, 李囡, 林新峰, 洪业, 杨志, 化学学报, 2014, 72, 427.)
doi: 10.6023/A13121248 |
|
| [66] |
Liu, X.; Wang, F.; Liu, L.; Li, T.; Zhong, X.; Lin, H.; Zhang, Y.; Xue, W. Biosens. Bioelectron. 2023, 222, 114995.
doi: 10.1016/j.bios.2022.114995 |
| [67] |
Xiao, W.; Wang, Y.; Lau, E. Y.; Luo, J.; Yao, N.; Shi, C.; Meza, L.; Tseng, H.; Maeda, Y.; Kumaresan, P.; Liu, R.; Lightstone, F. C.; Takada, Y.; Lam, K. S. Mol. Cancer Ther. 2010, 9, 2714.
doi: 10.1158/1535-7163.MCT-10-0308 |
| [68] |
Wang, Q.; Yan, H.; Jin, Y.; Wang, Z.; Huang, W.; Qiu, J.; Kang, F.; Wang, K.; Zhao, X.; Tian, J. Biomaterials 2018, 183, 173.
doi: 10.1016/j.biomaterials.2018.08.048 |
| [69] |
Liu, W.; Ma, H.; Li, F.; Cai, H.; Liang, R.; Chen, X.; Lan, T.; Yang, J.; Liao, J.; Yang, Y.; Liu, N. Bioorg. Med. Chem. 2022, 59, 116677.
doi: 10.1016/j.bmc.2022.116677 |
| [70] |
Nissen, N. I.; Karsdal, M.; Willumsen, N. J. Exp. Clin. Cancer Res. 2019, 38, 115.
doi: 10.1186/s13046-019-1110-6 pmid: 30841909 |
| [71] |
Xu, S.; Xu, H.; Wang, W.; Li, S.; Li, H.; Li, T.; Zhang, W.; Yu, X.; Liu, L. J. Transl. Med. 2019, 17, 309.
doi: 10.1186/s12967-019-2058-1 |
| [72] |
Leppänen, J.; Lindholm, V.; Isohookana, J.; Haapasaari, K. M.; Karihtala, P.; Lehenkari, P. P.; Saarnio, J.; Kauppila, J. H.; Karttunen, T. J.; Helminen, O.; Huhta, H. Pancreas 2019, 48, 43.
doi: 10.1097/MPA.0000000000001195 |
| [73] |
Van Obberghen-Schilling, E.; Tucker, R. P.; Saupe, F.; Gasser, I.; Cseh, B.; Orend, G. Int. J. Dev. Biol. 2011, 55, 511.
doi: 10.1387/ijdb.103243eo pmid: 21769776 |
| [74] |
Qiao, P.; Lu, Z. R. Adv. Exp. Med. Biol. 2020, 1245, 85.
|
| [75] |
Costantini, V.; Zacharski, L. R. Cancer Metastasis Rev. 1992, 11, 283.
doi: 10.1007/BF01307183 |
| [76] |
Wang, A. Y.; Mo, X.; Chen, C. S.; Yu, S. M. J. Am. Chem. Soc. 2005, 127, 4130.
doi: 10.1021/ja0431915 |
| [77] |
Bennink, L. L.; Li, Y.; Kim, B.; Shin, I. J.; San, B. H.; Zangari, M.; Yoon, D.; Yu, S. M. Biomaterials 2018, 183, 67.
doi: S0142-9612(18)30593-3 pmid: 30149231 |
| [78] |
Li, Y.; Foss, C. A.; Summerfield, D. D.; Doyle, J. J.; Torok, C. M.; Dietz, H. C.; Pomper, M. G.; Yu, S. M. Proc. Natl. Acad. Sci. U. S. A. 2012, 109, 14767.
doi: 10.1073/pnas.1209721109 |
| [79] |
Cai, X.; Wei, W.; Liu, Z.; Bai, Z; Lei, J.; Xiao, J. ACS Appl. Bio Mater. 2020, 3, 7492.
doi: 10.1021/acsabm.0c00710 |
| [80] |
Wei, W.; Li, D.; Cai, X.; Liu, Z.; Bai, Z.; Xiao, J. J. Mater. Chem. B 2020, 8, 10093.
doi: 10.1039/D0TB01691H |
| [81] |
Cai, X.; Wei, W.; Bi, Y.; Liu, Z.; Bai, Z.; Lei, J.; Xiao, J. Adv. Funct. Mater. 2020, 30, 2004532.
doi: 10.1002/adfm.v30.42 |
| [82] |
Kuhnast, B.; Bodenstein, C.; Haubner, R.; Wester, H. J.; Senekowitsch-Schmidtke, R.; Schwaiger, M.; Weber, W. A. Nucl. Med. Biol. 2004, 31, 337.
pmid: 15028246 |
| [83] |
Sprague, J. E.; Li, W. P.; Liang, K.; Achilefu, S.; Anderson, C. J. Nucl. Med. Biol. 2006, 33, 227.
doi: 10.1016/j.nucmedbio.2005.10.011 |
| [84] |
Zhu, L.; Wang, H.; Wang, L.; Wang, Y.; Jiang, K.; Li, C.; Ma, Q.; Gao, S.; Wang, L.; Li, W.; Cai, M.; Wang, H.; Niu, G.; Lee, S.; Yang, W.; Fang, X.; Chen, X. J. Control. Release 2011, 150, 248.
doi: 10.1016/j.jconrel.2011.01.032 |
| [85] |
Li, X.; Ma, Z.; Wang, H.; Ren, L.; Zhang, D.; Liang, W.; Zhang, G.; Zhang, J.; Yu, D.; Fang, X. Bioconjug. Chem. 2019, 30, 1507.
doi: 10.1021/acs.bioconjchem.9b00220 |
| [86] |
Ren, L.; Li, Q.; Ma, Z.; Wang, Y.; Li, H.; Shen, L.; Yu, J.; Fang, X. J. Mater. Chem. B 2018, 6, 7719.
doi: 10.1039/c8tb02025f pmid: 32254894 |
| [87] |
Starmans, L. W. E.; van Mourik, T.; Rossin, R.; Verel, I.; Nicolay, K.; Grüll, H. Mol. Pharm. 2015, 12, 1921.
doi: 10.1021/mp500673u |
| [88] |
Kim, M. Y.; Kim, O. R.; Choi, Y. S.; Lee, H.; Park, K.; Lee, C. T.; Kang, K. W.; Jeong, S. Mol. Cells 2012, 33, 71.
doi: 10.1007/S10059-012-2214-4 |
| [89] |
Han, Z.; Zhou, Z.; Shi, X.; Wang, J.; Wu, X.; Sun, D.; Chen, Y.; Zhu, H.; Magi-Galluzzi, C.; Lu, Z. R. Bioconjug. Chem. 2015, 26, 830.
doi: 10.1021/acs.bioconjchem.5b00178 |
| [90] |
Zhou, Z.; Qutaish, M.; Han, Z.; Schur, R. M.; Liu, Y.; Wilson, D. L.; Lu, Z. R. Nat. Commun. 2015, 6, 7984.
doi: 10.1038/ncomms8984 |
| [91] |
Zhou, Z.; Wu, X.; Kresak, A.; Griswold, M.; Lu, Z. R. Biomaterials 2013, 34, 7683.
doi: 10.1016/j.biomaterials.2013.06.057 |
| [92] |
Zhou, Z.; Lu, Z. R. Adv. Drug Deliver. Rev. 2017, 113, 24.
doi: 10.1016/j.addr.2016.07.012 |
| [93] |
Gao, C.; Tang, F.; Gong, G.; Zhang, J.; Hoi, M. P. M.; Lee, S. M. Y.; Wang, R. Nanoscale 2017, 9, 12533.
doi: 10.1039/C7NR03611F |
| [94] |
Dharmaratne, N. U.; Kaplan, A. R.; Glazer, P. M. Cells 2021, 10, 541.
doi: 10.3390/cells10030541 |
| [95] |
Reshetnyak, Y. K.; Yao, L.; Zheng, S.; Kuznetsov, S.; Engelman, D. M.; Andreev, O. A. Mol. Imaging Biol. 2011, 13, 1146.
doi: 10.1007/s11307-010-0457-z pmid: 21181501 |
| [96] |
Andreev, O. A.; Dupuy, A. D.; Segala, M.; Sandugu, S.; Serra, D. A.; Chichester, C. O.; Engelman, D. M.; Reshetnyak, Y. K. Proc. Natl. Acad. Sci. U. S. A. 2007, 104, 7893.
doi: 10.1073/pnas.0702439104 |
| [97] |
Demoin, D. W.; Wyatt, L. C.; Edwards, K. J.; Abdel-Atti, D.; Sarparanta, M.; Pourat, J.; Longo, V. A.; Carlin, S. D.; Engelman, D. M.; Andreev, O. A.; Reshetnyak, Y. K.; Viola-Villegas, N.; Lewis, J. S. Bioconjug. Chem. 2016, 27, 2014.
doi: 10.1021/acs.bioconjchem.6b00306 |
| [98] |
Crawford, T.; Moshnikova, A.; Roles, S.; Weerakkody, D.; DuPont, M.; Carter, L. M.; Shen, J.; Engelman, D. M.; Lewis, J. S.; Andreev, O. A.; Reshetnyak, Y. K. Sci. Rep. 2020, 10, 18356.
doi: 10.1038/s41598-020-75443-5 pmid: 33110131 |
| [99] |
Moyer, T. J.; Finbloom, J. A.; Chen, F.; Toft, D. J.; Cryns, V. L.; Stupp, S. I. J. Am. Chem. Soc. 2014, 136, 14746.
doi: 10.1021/ja5042429 |
| [100] |
Myochin, T.; Hanaoka, K.; Komatsu, T.; Terai, T.; Nagano, T. J. Am. Chem. Soc. 2012, 134, 13730.
doi: 10.1021/ja303931b pmid: 22830429 |
| [101] |
Liu, Y.; Zhang, D.; Qiao, Z. Y.; Qi, G. B.; Liang, X. J.; Chen, X. G.; Wang, H. Adv. Mater. 2015, 27, 5034.
doi: 10.1002/adma.201501502 |
| [102] |
Sun, L.; Xie, S.; Qi, J.; Liu, E.; Liu, D.; Liu, Q.; Chen, S.; He, H.; Yang, V. C. ACS Appl. Mater. Interfaces 2017, 9, 39209.
doi: 10.1021/acsami.7b12918 |
| [103] |
Ma, P.; Chen, J.; Bi, X.; Li, Z.; Gao, X.; Li, H.; Zhu, H.; Huang, Y.; Qi, J.; Zhang, Y. ACS Appl. Mater. Interfaces 2018, 10, 12351.
doi: 10.1021/acsami.7b18437 |
| [104] |
Kasten, B. B.; Jiang, K.; Cole, D.; Jani, A.; Udayakumar, N.; Gillespie, G. Y.; Lu, G.; Dai, T.; Rosenthal, E. L.; Markert, J. M.; Rao, J.; Warram, J. M. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1412.
doi: 10.1007/s00259-019-04607-x |
| [105] |
Chen, X.; Lee, D.; Yu, S.; Kim, G.; Lee, S.; Cho, Y.; Jeong, H.; Nam, K. T.; Yoon, J. Biomaterials 2017, 122, 130.
doi: 10.1016/j.biomaterials.2017.01.020 |
| [106] |
Kisin-Finfer, E.; Ferber, S.; Blau, R.; Satchi-Fainaro, R.; Shabat, D. Bioorg. Med. Chem. Lett. 2014, 24, 2453.
doi: 10.1016/j.bmcl.2014.04.022 pmid: 24767838 |
| [107] |
Shi, W.; Ogbomo, S. M.; Wagh, N. K.; Zhou, Z.; Jia, Y.; Brusnahan, S. K.; Garrison, J. C. Biomaterials 2014, 35, 5760.
doi: 10.1016/j.biomaterials.2014.03.056 |
| [108] |
Kim, J.; Won, Y.; Goh, S. H.; Choi, Y. J. Mater. Chem. B 2016, 4, 6787.
doi: 10.1039/C6TB01519K |
| [109] |
Cai, Z.; Wang, A.; Wang, Y.; Qiu, Z.; Li, Y.; Yan, H.; Fu, M.; Liu, M.; Yu, Y.; Gao, F. Anal. Chem. 2022, 94, 9715.
doi: 10.1021/acs.analchem.2c01159 |
| [110] |
Wang, X.; Xia, Y.; Liu, Y.; Qi, W.; Sun, Q.; Zhao, Q.; Tang, B. Chemistry 2012, 18, 7189.
|
| [111] |
Huang, S.; Shao, K.; Liu, Y.; Kuang, Y.; Li, J.; An, S.; Guo, Y.; Ma, H.; Jiang, C. ACS Nano 2013, 7, 2860.
doi: 10.1021/nn400548g |
| [112] |
Qiao, Z. Y.; Zhao, W. J.; Gao, Y. J.; Cong, Y.; Zhao, L.; Hu, Z.; Wang, H. ACS Appl. Mater. Interfaces 2017, 9, 30426.
doi: 10.1021/acsami.7b09033 |
| [113] |
Mohtavinejad, N.; Shafiee Ardestani, M.; Khalaj, A.; Pormohammad, A.; Najafi, R.; Bitarafan-Rajabi, A.; Hajiramezanali, M.; Amanlou, M. Life Sci. 2020, 258, 118206.
doi: 10.1016/j.lfs.2020.118206 |
| [114] |
Khalily, M. P.; Soydan, M. Chem. Biol. Drug Des. 2023, 101, 772.
doi: 10.1111/cbdd.v101.3 |
| [1] | 杜思南, 赵丽曼, 张泽新, 陈国颂. 甘露糖修饰的微马达的制备及其免疫功能初探★[J]. 化学学报, 2023, 81(7): 741-748. |
| [2] | 张沛森, 荆莉红. 肿瘤病理可视化纳米探针的研究进展※[J]. 化学学报, 2022, 80(6): 805-816. |
| [3] | 郭彩霞, 马小杰, 王博. 金属有机框架基复合材料的制备及其光热性能研究[J]. 化学学报, 2021, 79(8): 967-985. |
| [4] | 易荣楠, 吴燕. 表面增强拉曼光谱技术在microRNA检测中的研究进展[J]. 化学学报, 2021, 79(6): 694-704. |
| [5] | 王涛, 赵璐, 王科伟, 白云峰, 冯锋. 共价有机框架的合成及其在肿瘤治疗中的应用研究进展[J]. 化学学报, 2021, 79(5): 600-613. |
| [6] | 蔡政, 张颖雯, 姜立萍, 朱俊杰. 基于类芬顿反应的Mn3O4/DOX@Lip纳米递药体系的构建及应用[J]. 化学学报, 2021, 79(4): 481-489. |
| [7] | 李佳欣, 李蓓, 王纪康, 何蕾, 赵宇飞. 水滑石(LDHs)及其衍生物在生物医药领域的研究进展[J]. 化学学报, 2021, 79(3): 238-256. |
| [8] | 廖妮, 钟霞, 梁文斌, 袁若, 卓颖. ECL金属-有机框架(MOF)生物传感平台用于肿瘤细胞分泌H2O2的测定[J]. 化学学报, 2021, 79(10): 1257-1264. |
| [9] | 张留伟, 陈麒先, 王静云. 活性氧响应型抗肿瘤前药研究进展[J]. 化学学报, 2020, 78(7): 642-656. |
| [10] | 闫涛, 刘振华, 宋昕玥, 张书圣. 肿瘤微环境刺激响应型上转换光动力诊疗体系的构建和发展[J]. 化学学报, 2020, 78(7): 657-669. |
| [11] | 刘启雁, 蔡戴宏, 戚永育, 乐学义. 司帕沙星及均三嗪衍生物铜(II)配合物与DNA作用及其抗肿瘤活性[J]. 化学学报, 2020, 78(3): 263-270. |
| [12] | 马秋琳, 冯楠, 鞠熀先. 前列腺癌相关肿瘤标志物分析方法的研究进展[J]. 化学学报, 2020, 78(11): 1213-1222. |
| [13] | 曹萌轩, 代晓光, 陈贝贝, 赵娜娜, 徐福建. 纳米材料与细菌结合应用于肿瘤治疗[J]. 化学学报, 2020, 78(10): 1054-1063. |
| [14] | 金鑫, 王晓英. 口腔癌相关唾液肿瘤生物标志物的分析检测研究进展[J]. 化学学报, 2019, 77(4): 340-350. |
| [15] | 熊麟, 凡勇, 张凡. 稀土纳米晶用于近红外区活体成像和传感研究进展[J]. 化学学报, 2019, 77(12): 1239-1249. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||