化学学报 ›› 2024, Vol. 82 ›› Issue (6): 577-588.DOI: 10.6023/A24040122 上一篇 下一篇
研究论文
投稿日期:2024-04-08
发布日期:2024-06-04
基金资助:Received:2024-04-08
Published:2024-06-04
Contact:
* E-mail: Supported by:文章分享
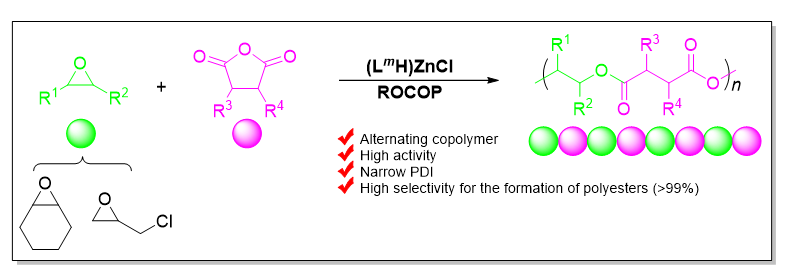
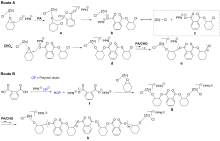
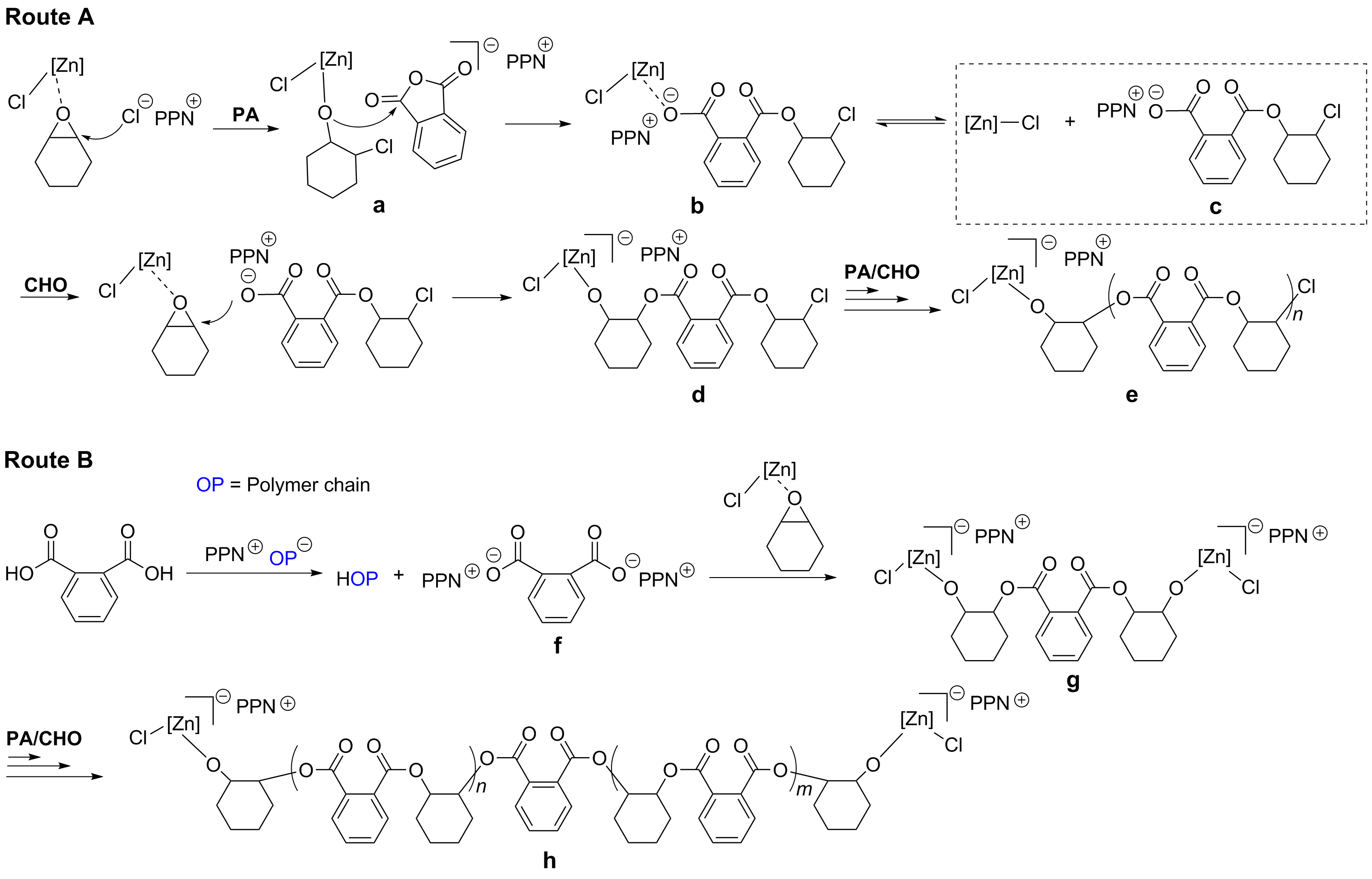
本工作合成了一系列基于爪形氨基酚氧基配体的新型锌氯化物, 均通过1H NMR、13C NMR以及元素分析等进行了表征, 且典型络合物通过X-ray单晶衍射分析确定了其空间配位结构. 利用上述络合物实现了催化环氧环己烷、外消旋环氧氯丙烷和邻苯二甲酸酐的共聚反应. 在共聚过程中均表现出较高催化活性以及高酯链节选择性(>99%), 得到分子量分布较窄的交替共聚物. 典型络合物Zn1在80 ℃下催化环氧环己烷和邻苯二甲酸酐共聚时, TOFPA值可达到490 h-1, 在相同条件下催化外消旋环氧氯丙烷和邻苯二甲酸酐共聚时, TOFPA值可达到848 h-1. 研究表明, 共聚反应可能通过环氧化物在亲核试剂进攻下开环引发, 经后续重复的酸酐和环氧化物交替插入得到高酯链节含量共聚物.
王海成, 马海燕. 爪形氨基酚氧基锌氯化物催化环氧化物和酸酐共聚研究[J]. 化学学报, 2024, 82(6): 577-588.
Haicheng Wang, Haiyan Ma. Ring-opening Copolymerization of Epoxides and Anhydride Mediated by Claw-type Aminophenolate Zinc Chlorides[J]. Acta Chimica Sinica, 2024, 82(6): 577-588.
| Entry | Cat. | Temp/℃ | Time/min | Conv.b/% | TOFPAc/h-1 | Esterd/% | Mn,calcde/×103 | Mnf/×103 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn1 | 80 | 22 | 90 | 490 | 99 | 8.9 | 5.5 | 1.16 |
| 2 | Zn2 | 25 | 31 h | 85 | 5 | 99 | 8.4 | 4.9 | 1.21 |
| 3g | 80 | 10 h | 20 | 4 | 12 | — | — | — | |
| 4h | 80 | 125 | 89 | 85 | 96 | 43.8 | 6.5 | 1.17 | |
| 5 | 80 | 26 | 87 | 402 | 99 | 8.6 | 5.0 | 1.22 | |
| 6 | Zn3 | 80 | 28 | 91 | 400 | 99 | 9.0 | 5.6 | 1.23 |
| 7 | Zn4 | 80 | 29 | 93 | 384 | 99 | 9.2 | 5.7 | 1.14 |
| 8 | Zn5 | 80 | 34 | 87 | 307 | 99 | 8.6 | 4.9 | 1.23 |
| 9 | Zn6 | 80 | 21 | 82 | 468 | 99 | 8.1 | 4.3 | 1.24 |
| 10 | Zn7 | 80 | 26 | 84 | 388 | 99 | 8.3 | 4.7 | 1.24 |
| 11 | Zn8 | 80 | 27 | 82 | 364 | 99 | 8.1 | 4.3 | 1.23 |
| Entry | Cat. | Temp/℃ | Time/min | Conv.b/% | TOFPAc/h-1 | Esterd/% | Mn,calcde/×103 | Mnf/×103 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn1 | 80 | 22 | 90 | 490 | 99 | 8.9 | 5.5 | 1.16 |
| 2 | Zn2 | 25 | 31 h | 85 | 5 | 99 | 8.4 | 4.9 | 1.21 |
| 3g | 80 | 10 h | 20 | 4 | 12 | — | — | — | |
| 4h | 80 | 125 | 89 | 85 | 96 | 43.8 | 6.5 | 1.17 | |
| 5 | 80 | 26 | 87 | 402 | 99 | 8.6 | 5.0 | 1.22 | |
| 6 | Zn3 | 80 | 28 | 91 | 400 | 99 | 9.0 | 5.6 | 1.23 |
| 7 | Zn4 | 80 | 29 | 93 | 384 | 99 | 9.2 | 5.7 | 1.14 |
| 8 | Zn5 | 80 | 34 | 87 | 307 | 99 | 8.6 | 4.9 | 1.23 |
| 9 | Zn6 | 80 | 21 | 82 | 468 | 99 | 8.1 | 4.3 | 1.24 |
| 10 | Zn7 | 80 | 26 | 84 | 388 | 99 | 8.3 | 4.7 | 1.24 |
| 11 | Zn8 | 80 | 27 | 82 | 364 | 99 | 8.1 | 4.3 | 1.23 |
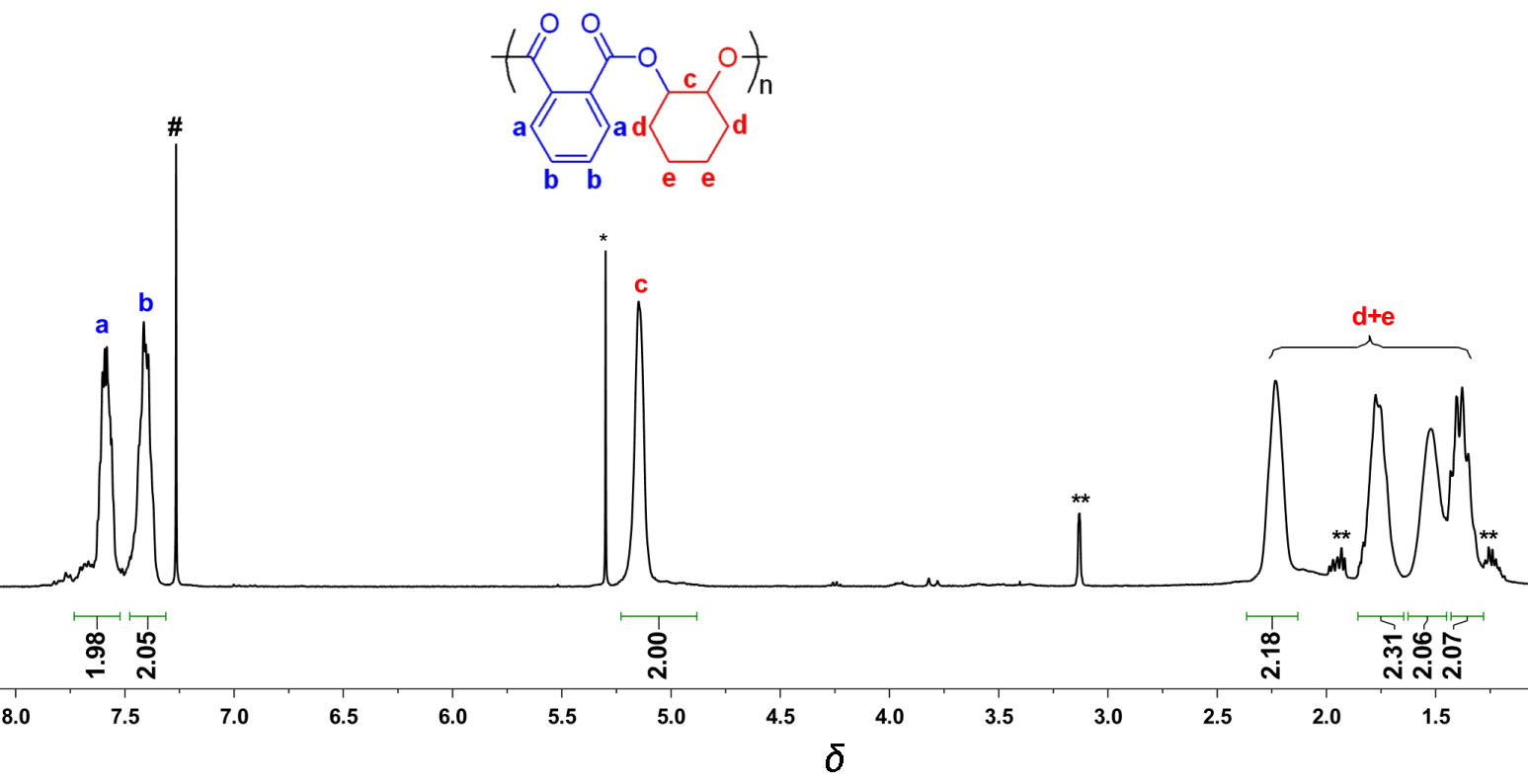

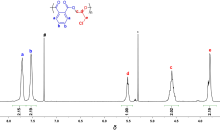
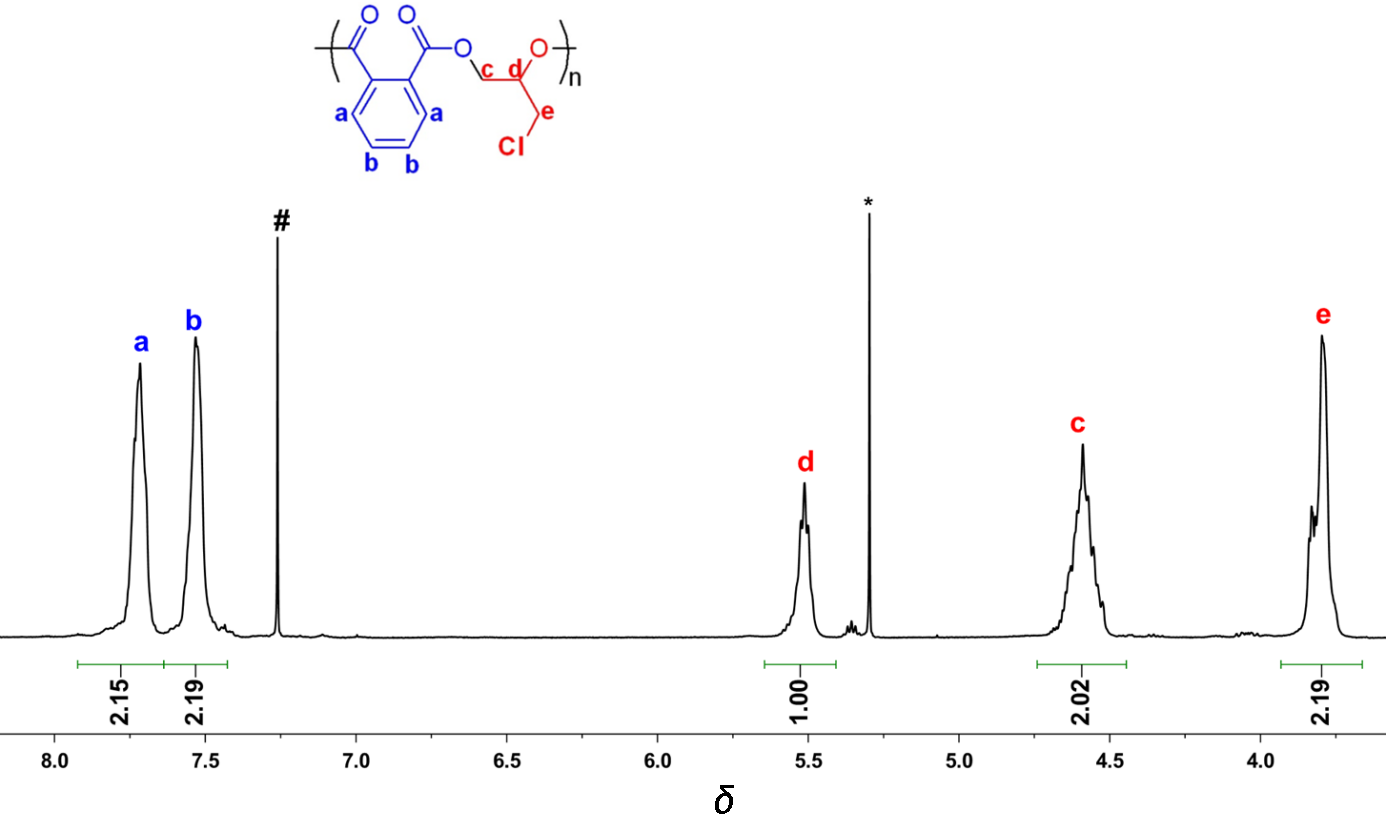
| Run | Cat. | Temp/℃ | Time/min | Conv.b/% | TOFPAc/h-1 | Esterd/% | Mn,calcde/×103 | Mnf/×103 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn1 | 80 | 14 | 99 | 848 | 99 | 9.5 | 2.9 | 1.49 |
| 2 | Zn2 | 25 | 24 h | 87 | 7 | 99 | 8.4 | 2.1 | 1.37 |
| 3 | 80 | 14 | 87 | 745 | 99 | 8.4 | 2.3 | 1.44 | |
| 4 | Zn3 | 80 | 15 | 89 | 710 | 99 | 8.5 | 2.3 | 1.37 |
| 5 | Zn4 | 80 | 16 | 89 | 668 | 99 | 8.5 | 2.5 | 1.48 |
| 6 | Zn5 | 80 | 17 | 85 | 600 | 99 | 8.4 | 2.1 | 1.39 |
| 7 | Zn6 | 80 | 12 | 80 | 800 | 99 | 7.7 | 1.9 | 1.45 |
| 8 | Zn7 | 80 | 15 | 90 | 720 | 99 | 8.6 | 2.2 | 1.43 |
| 9 | Zn8 | 80 | 18 | 90 | 640 | 99 | 8.6 | 2.3 | 1.36 |
| Run | Cat. | Temp/℃ | Time/min | Conv.b/% | TOFPAc/h-1 | Esterd/% | Mn,calcde/×103 | Mnf/×103 | Mw/Mnf |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn1 | 80 | 14 | 99 | 848 | 99 | 9.5 | 2.9 | 1.49 |
| 2 | Zn2 | 25 | 24 h | 87 | 7 | 99 | 8.4 | 2.1 | 1.37 |
| 3 | 80 | 14 | 87 | 745 | 99 | 8.4 | 2.3 | 1.44 | |
| 4 | Zn3 | 80 | 15 | 89 | 710 | 99 | 8.5 | 2.3 | 1.37 |
| 5 | Zn4 | 80 | 16 | 89 | 668 | 99 | 8.5 | 2.5 | 1.48 |
| 6 | Zn5 | 80 | 17 | 85 | 600 | 99 | 8.4 | 2.1 | 1.39 |
| 7 | Zn6 | 80 | 12 | 80 | 800 | 99 | 7.7 | 1.9 | 1.45 |
| 8 | Zn7 | 80 | 15 | 90 | 720 | 99 | 8.6 | 2.2 | 1.43 |
| 9 | Zn8 | 80 | 18 | 90 | 640 | 99 | 8.6 | 2.3 | 1.36 |
| [1] |
Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Chem. Rev. 2004, 104, 6147.
doi: 10.1021/cr040002s pmid: 15584698 |
| [2] |
Williams, C. K. Chem. Soc. Rev. 2007, 36, 1573.
|
| [3] |
Williams, C. K.; Hillmyer, M. Polym. Rev. 2008, 48, 1.
|
| [4] |
Vroman, I.; Tighzert, L. Materials 2009, 2, 307.
|
| [5] |
Thomas, C. M. Chem. Soc. Rev. 2010, 39, 165.
|
| [6] |
Vilela, C.; Sousa, A. F.; Fonseca, A. C.; Serra, A. C.; Coelho, J. F. J.; Freire, C. S. R.; Silvestre, A. J. D. Polym. Chem. 2014, 5, 3119.
|
| [7] |
Zhou, Z.; Liu, S.; Lang, T.; Gao, F.; Wang, X. Acta Polym. Sin. 2024, 55, 396. (in Chinese)
|
|
(周振震, 刘顺杰, 郎涛涛, 高凤翔, 王献红, 高分子学报, 2024, 55, 396.)
|
|
| [8] |
Sanford, M. J.; Peña Carrodeguas, L.; Van Zee, N. J.; Kleij, A. W.; Coates, G. W. Macromolecules 2016, 49, 6394.
|
| [9] |
Hu, L.-F.; Zhang, C.-J.; Wu, H.-L.; Yang, J.-L.; Liu, B.; Duan, H.-Y.; Zhang, X.-H. Macromolecules 2018, 51, 3126.
|
| [10] |
Ji, H.-Y.; Wang, B.; Pan, L.; Li, Y.-S. Angew. Chem. Int. Ed. 2018, 57, 16888.
|
| [11] |
Liu, Y.; Guo, J.-Z.; Lu, H.-W.; Wang, H.-B.; Lu, X.-B. Macromolecules 2018, 51, 771.
|
| [12] |
Stößer, T.; Williams, C. K. Angew. Chem. Int. Ed. 2018, 57, 6337.
doi: 10.1002/anie.201801400 pmid: 29518288 |
| [13] |
Liu, F.-P.; Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Macromolecules 2019, 52, 5652.
|
| [14] |
Zhou, Y.; Hu, C.; Zhang, T.; Xu, X.; Duan, R.; Luo, Y.; Sun, Z.; Pang, X.; Chen, X. Macromolecules 2019, 52, 3462.
|
| [15] |
Chen, X.; Chen, G.; Tao, Y.; Wang, Y.; Lu, X.; Zhang, L.; Zhu, J.; Zhang, J.; Wang, X. Acta Polym. Sin. 2019, 50, 1068. (in Chinese)
|
|
(陈学思, 陈国强, 陶友华, 王玉忠, 吕小兵, 张立群, 朱锦, 张军, 王献红, 高分子学报, 2019, 50, 1068.)
|
|
| [16] |
Lidston, C. A. L.; Abel, B. A.; Coates, G. W. J. Am. Chem. Soc. 2020, 142, 20161.
doi: 10.1021/jacs.0c10014 pmid: 33176426 |
| [17] |
Fang, J.; Hu, F. Chin. J. Catal. 2002, 23, 88. (in Chinese)
|
|
(房江华, 胡富陶, 催化学报, 2002, 23, 88.)
|
|
| [18] |
Huang, Y.; Qi, G.; Feng, L. Chin. J. Catal. 2002, 23, 113. (in Chinese)
|
|
(黄亦军, 戚国荣, 封麟先, 催化学报, 2002, 23, 113.)
|
|
| [19] |
Hua, Z.; Chen, S.; Fang, Z.; Qi, G. Acta Polym. Sin. 2004, 4, 551. (in Chinese)
|
|
(华正江, 陈上, 方佐, 戚国荣, 高分子学报, 2004, 4, 551.)
|
|
| [20] |
Huijser, S.; HosseiniNejad, E.; Sablong, R.; de Jong, C.; Koning, C. E.; Duchateau, R. Macromolecules 2011, 44, 1132.
|
| [21] |
Longo, J. M.; DiCiccio, A. M.; Coates, G. W. J. Am. Chem. Soc. 2014, 136, 15897.
|
| [22] |
Van Zee, N. J.; Coates, G. W. Chem. Commun. 2014, 50, 6322.
|
| [23] |
Baumgartner, R.; Song, Z.; Zhang, Y.; Cheng, J. Polym. Chem. 2015, 6, 3586.
|
| [24] |
Si, G.; Zhang, L.; Han, B.; Duan, Z.; Li, B.; Dong, J.; Li, X.; Liu, B. Polym. Chem. 2015, 6, 6372.
|
| [25] |
Zhu, Y.; Romain, C.; Poirier, V.; Williams, C. K. Macromolecules 2015, 48, 2407.
|
| [26] |
DiCiccio, A. M.; Longo, J. M.; Rodríguez-Calero, G. G.; Coates, G. W. J. Am. Chem. Soc. 2016, 138, 7107.
doi: 10.1021/jacs.6b03113 pmid: 27171536 |
| [27] |
Han, B.; Zhang, L.; Yang, M.; Liu, B.; Dong, X.; Theato, P. Macromolecules 2016, 49, 6232.
|
| [28] |
Fieser, M. E.; Sanford, M. J.; Mitchell, L. A.; Dunbar, C. R.; Mandal, M.; Van Zee, N. J.; Urness, D. M.; Cramer, C. J.; Coates, G. W.; Tolman, W. B. J. Am. Chem. Soc. 2017, 139, 15222.
doi: 10.1021/jacs.7b09079 pmid: 28984455 |
| [29] |
Zhou, Y.; Duan, R.; Li, X.; Pang, X.; Wang, X.; Chen, X. Chem. -Asian J. 2017, 12, 3135.
|
| [30] |
Bester, K.; Bukowska, A.; Myśliwiec, B.; Hus, K.; Tomczyk, D.; Urbaniak, P.; Bukowski, W. Polym. Chem. 2018, 9, 2147.
|
| [31] |
Chen, T. T. D.; Zhu, Y.; Williams, C. K. Macromolecules 2018, 51, 5346.
|
| [32] |
Hiranoi, Y.; Nakano, K. Beilstein J. Org. Chem. 2018, 14, 2779.
doi: 10.3762/bjoc.14.255 pmid: 30498527 |
| [33] |
Martínez, J.; Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Alonso-Moreno, C.; Sánchez-Barba, L. F.; Fernandez-Baeza, J.; Rodríguez, A. M.; Rodríguez-Diéguez, A.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. Dalton Trans. 2018, 47, 7471.
doi: 10.1039/c8dt01553h pmid: 29786721 |
| [34] |
Sanford, M. J.; Van Zee, N. J.; Coates, G. W. Chem. Sci. 2018, 9, 134.
doi: 10.1039/c7sc03643d pmid: 29629081 |
| [35] |
Ji, H.-Y.; Song, D.-P.; Wang, B.; Pan, L.; Li, Y.-S. Green Chem. 2019, 21, 6123.
|
| [36] |
Kernbichl, S.; Reiter, M.; Mock, J.; Rieger, B. Macromolecules 2019, 52, 8476.
|
| [37] |
Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. Proc. Natl. Acad. Sci. 2020, 117, 15429.
|
| [38] |
Lin, L.; Liang, J.; Xu, Y.; Wang, S.; Xiao, M.; Sun, L.; Meng, Y. Green Chem. 2019, 21, 2469.
|
| [39] |
Li, J.; Ren, B.-H.; Chen, S.-Y.; He, G.-H.; Liu, Y.; Ren, W.-M.; Zhou, H.; Lu, X.-B. ACS Catal. 2019, 9, 1915.
|
| [40] |
Li, W.-B.; Liu, Y.; Lu, X.-B. Organometallics 2020, 39, 1628.
|
| [41] |
Ryu, H. K.; Bae, D. Y.; Lim, H.; Lee, E.; Son, K.-S. Polym. Chem. 2020, 11, 3756.
|
| [42] |
Shi, D.; Li, L.; Wen, Y.; Yang, Q.; Duan, Z. Polym. Int. 2020, 69, 513.
|
| [43] |
Sulley, G. S.; Gregory, G. L.; Chen, T. T. D.; Peña Carrodeguas, L.; Trott, G.; Santmarti, A.; Lee, K.-Y.; Terrill, N. J.; Williams, C. K. J. Am. Chem. Soc. 2020, 142, 4367.
doi: 10.1021/jacs.9b13106 pmid: 32078313 |
| [44] |
Wang, L.; Zhang, J.; Zhao, N.; Ren, C.; Liu, S.; Li, Z. ACS Macro Lett. 2020, 9, 1398.
|
| [45] |
Zhu, S.; Wang, Y.; Ding, W.; Zhou, X.; Liao, Y.; Xie, X. Polym. Chem. 2020, 11, 1691.
|
| [46] |
Li, S.; Wang, Y.; Ji, H.; Chen, C.; Chen, X.; Pan, L.; Wang, B. Acta Polym. Sin. 2020, 51, 1039. (in Chinese)
|
|
(李帅, 王昱博, 季鹤源, 陈崇民, 陈晓璐, 潘莉, 王彬, 高分子学报, 2020, 51, 1039.)
|
|
| [47] |
Cui, L.; Ren, B.-H.; Lu, X.-B. J. Polym. Sci. 2021, 59, 1821.
|
| [48] |
Diment, W. T.; Stößer, T.; Kerr, R. W. F.; Phanopoulos, A.; Durr, C. B.; Williams, C. K. Catal. Sci. Technol. 2021, 11, 1737.
|
| [49] |
Aida, T.; Inoue, S. J. Am. Chem. Soc. 1985, 107, 1358.
|
| [50] |
Jeon, J. Y.; Eo, S. C.; Varghese, J. K.; Lee, B. Y. Beilstein J. Org. Chem. 2014, 10, 1787.
|
| [51] |
Diment, W. T.; Gregory, G. L.; Kerr, R. W. F.; Phanopoulos, A.; Buchard, A.; Williams, C. K. ACS Catal. 2021, 11, 12532.
|
| [52] |
Liu, D.-F.; Wu, L.-Y.; Feng, W.-X.; Zhang, X.-M.; Wu, J.; Zhu, L.-Q.; Fan, D.-D.; Lü, X.-Q.; Shi, Q. J. Mol. Catal. A-Chem. 2014, 382, 136.
|
| [53] |
Saini, P. K.; Romain, C.; Zhu, Y.; Williams, C. K. Polym. Chem. 2014, 5, 6068.
|
| [54] |
Thevenon, A.; Garden, J. A.; White, A. J. P.; Williams, C. K. Inorg. Chem. 2015, 54, 11906.
doi: 10.1021/acs.inorgchem.5b02233 pmid: 26605983 |
| [55] |
Ji, H.-Y.; Wang, B.; Pan, L.; Li, Y.-S. Green Chem. 2018, 20, 641.
|
| [56] |
Wang, L.; Ma, H. Dalton Trans. 2010, 39, 7897.
|
| [57] |
Darensbourg, D. J.; Poland, R. R.; Escobedo, C. Macromolecules 2012, 45, 2242.
|
| [58] |
Li, J.; Liu, Y.; Ren, W.-M.; Lu, X.-B. J. Am. Chem. Soc. 2016, 138, 11493.
|
| [59] |
Stößer, T.; Mulryan, D.; Williams, C. K. Angew. Chem. Int. Ed. 2018, 57, 16893.
doi: 10.1002/anie.201810245 pmid: 30370965 |
| [60] |
Abel, B. A.; Lidston, C. A. L.; Coates, G. W. J. Am. Chem. Soc. 2019, 141, 12760.
|
| [61] |
Ryu, H. K.; Cha, J.; Yu, N.; Lee, E.; Son, K. S. Inorg. Chem. Commun. 2020, 122, 108278.
|
| [62] |
Wang, H.; Ma, H. Chem. Commun. 2013, 49, 8686.
|
| [63] |
Mundil, R.; Hošt’álek, Z.; Šeděnková, I.; Merna, J. Macromol. Res. 2015, 23, 161.
|
| [64] |
Martínez de Sarasa Buchaca, M.; de la Cruz-Martínez, F.; Martínez, J.; Alonso-Moreno, C.; Fernández-Baeza, J.; Tejeda, J.; Niza, E.; Castro-Osma, J. A.; Otero, A.; Lara-Sánchez, A. ACS Omega 2018, 3, 17581.
doi: 10.1021/acsomega.8b02759 pmid: 31458360 |
| [65] |
Chen, C.-M.; Xu, X.; Ji, H.-Y.; Wang, B.; Pan, L.; Luo, Y.; Li, Y.-S. Macromolecules 2021, 54, 713.
|
| [66] |
Martínez, G.; Pedrosa, S.; Tabernero, V.; Mosquera, M. E. G.; Cuenca, T. Organometallics 2008, 27, 2300.
|
| [67] |
Ling, J.; You, L.; Wang, Y.; Shen, Z. J. Appl. Polym. Sci. 2012, 124, 2537.
|
| [68] |
Ambrose, K.; Murphy, J. N.; Kozak, C. M. Macromolecules 2019, 52, 7403.
doi: 10.1021/acs.macromol.9b01381 |
| [69] |
Chen, P.; Chisholm, M. H.; Gallucci, J. C.; Zhang, X.; Zhou, Z. Inorg. Chem. 2005, 44, 2588.
|
| [70] |
Chisholm, M. H.; Navarro-Llobet, D.; Simonsick, W. J. Macromolecules 2001, 34, 8851.
|
| [71] |
Hošťálek, Z.; Trhlíková, O.; Walterová, Z.; Martinez, T.; Peruch, F.; Cramail, H.; Merna, J. Eur. Polym. J. 2017, 88, 433.
|
| [72] |
Xie, R.; Zhang, Y.-Y.; Yang, G.-W.; Zhu, X.-F.; Li, B.; Wu, G.-P. Angew. Chem. Int. Ed. 2021, 60, 19253.
|
| [73] |
Li, J.; Ren, B.-H.; Wan, Z.-Q.; Chen, S.-Y.; Liu, Y.; Ren, W.-M.; Lu, X.-B. J. Am. Chem. Soc. 2019, 141, 8937.
|
| [74] |
Huang, M.; Pan, C.; Ma, H. Dalton Trans. 2015, 44, 12420.
doi: 10.1039/c5dt00158g pmid: 25997024 |
| [75] |
Yang, Y.; Wang, H.; Ma, H. Inorg. Chem. 2015, 54, 5839.
doi: 10.1021/acs.inorgchem.5b00558 pmid: 25996447 |
| [1] | 易敬霖, 陈茂. 三氟氯乙烯与甲基异丙烯基醚的光诱导共聚反应★[J]. 化学学报, 2024, 82(2): 126-131. |
| [2] | 李西安, 李孝坤. 基于温度诱导相转变共聚物和导电聚合物的自隔断超级电容器[J]. 化学学报, 2023, 81(5): 511-519. |
| [3] | 汪洋, 向焌钧, 葛从伍, 高希珂. 2,6-薁和3,4-丙撑二氧噻吩共聚物的主链结构调控及性质研究★[J]. 化学学报, 2023, 81(10): 1341-1349. |
| [4] | 李卫华. 桥连对嵌段共聚物自组装的调控[J]. 化学学报, 2021, 79(2): 133-138. |
| [5] | 江金辉, 朱云卿, 杜建忠. 开环聚合诱导自组装的挑战与展望[J]. 化学学报, 2020, 78(8): 719-724. |
| [6] | 贾晓燕, 李振环. NaZnPO4催化碳酸二甲酯和丙氨酸“一锅法”合成N-羧基丙氨酸酸酐[J]. 化学学报, 2020, 78(6): 540-546. |
| [7] | 李荣烨, Khiman Mehul, 盛力, 孙静. 两亲性聚氨基酸三嵌段共聚物构筑pH/溶剂可控多级纳米结构[J]. 化学学报, 2020, 78(11): 1235-1239. |
| [8] | 崔惠娜, 邱枫, 彭娟. 一种基于聚噻吩-聚硒吩全共轭嵌段共聚物的合成及性质研究[J]. 化学学报, 2018, 76(9): 691-700. |
| [9] | 布美热木·克力木, 马海燕. 非对称β-二亚氨基镁络合物催化丙交酯、己内酯开环聚合/共聚研究[J]. 化学学报, 2018, 76(2): 121-132. |
| [10] | 郑剑峰, 谢志强, 陈欣健, 黄培强. 酰胺的直接转化:仲酰胺与丹尼谢夫斯基双烯的还原环加成反应[J]. 化学学报, 2015, 73(7): 705-715. |
| [11] | 丁妍春, 俞燕蕾, 韦嘉. 不同亲疏水比例的光响应性嵌段共聚物的合成及溶液组装行为研究[J]. 化学学报, 2014, 72(5): 602-608. |
| [12] | 郝莹, 张洋, 何金林, 尚修娟, 张明祖, 倪沛红. 半乳糖胺修饰阳离子型刷形嵌段共聚物的合成与表征[J]. 化学学报, 2014, 72(5): 569-576. |
| [13] | 江昱倩, 徐海华, 赵妮, 彭谦, 帅志刚. 给受共聚物链上与链间极化子的光谱特性[J]. 化学学报, 2014, 72(2): 201-207. |
| [14] | 夏彬凯, 李卫华, 邱枫. 对称AB两嵌段共聚物在均聚物C中的自组装[J]. 化学学报, 2014, 72(1): 30-34. |
| [15] | 张广萌, 杨继萍, 陈功. 苯胺四聚体-聚乙二醇-苯胺四聚体嵌段共聚物薄膜的自组装[J]. 化学学报, 2014, 72(1): 83-88. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
